How MCP Can Break Down Data Silos and Transform Collaboration in Life Sciences R&D?
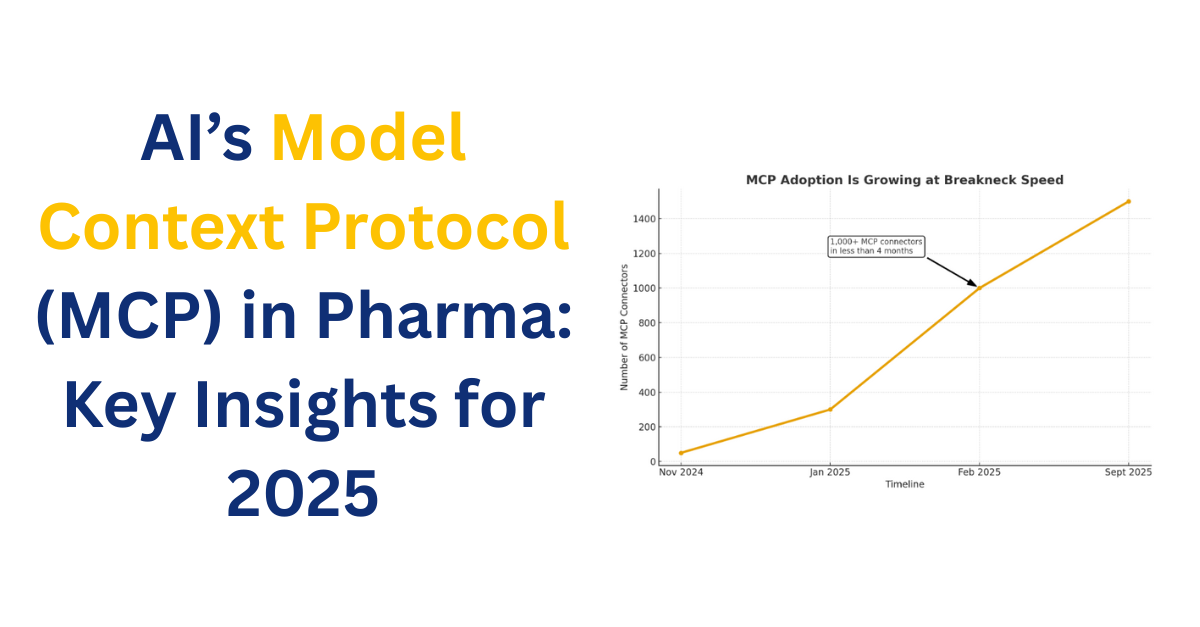
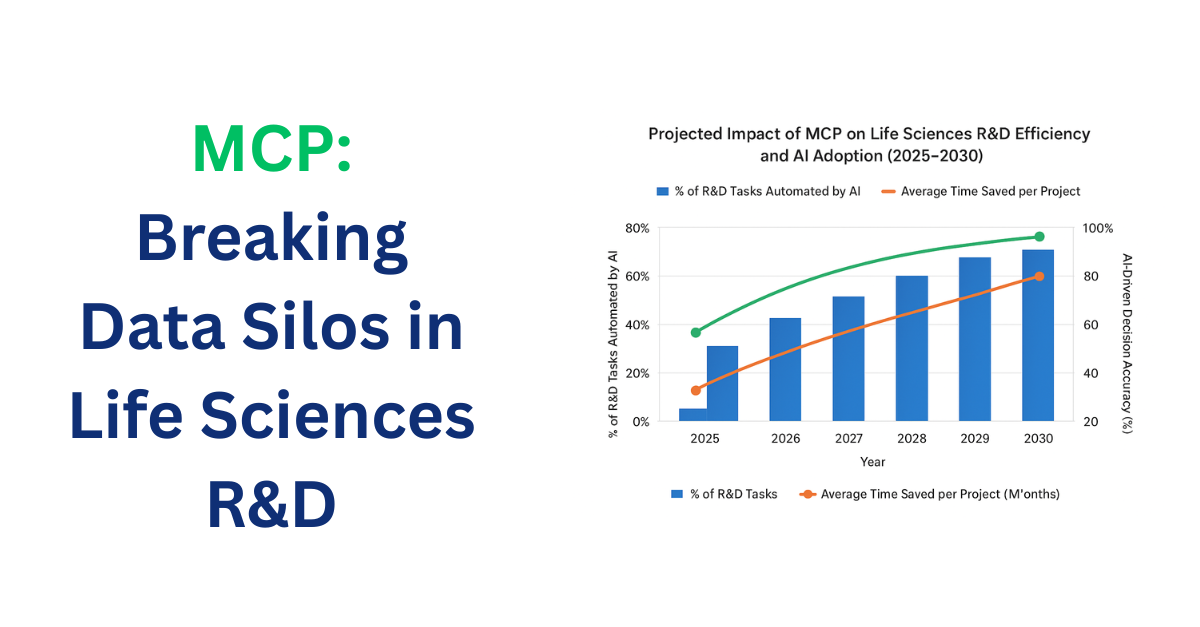
tl;dr: Data silos slow life sciences R&D, causing inefficiencies and errors. The Model Context Protocol (MCP) enables AI systems to access and integrate data across departments securely, breaking silos, streamlining workflows, and accelerating research and decision-making. Early adoption of MCP is essential for faster, smarter, and more collaborative R&D. The Challenge of Data Silos in […]