The Model Context Protocol (MCP) is a rising open standard that lets AI systems plug securely into data, apps, and services the way a single physical port lets you plug in many devices. For pharma and life sciences, MCP can break down data silos, speed trial design and monitoring, improve regulatory visibility, and enable new commercial and patient-facing experiences, but it also creates new security, governance, and validation challenges. Senior leaders should treat MCP as a strategic lever: prioritize high-value pilots, shore up data governance and security, and consider interim AI leadership to translate the technical standard into business outcomes.
Why MCP Belongs in the Boardroom
Many executives assume protocols are purely an engineering concern—but in pharma, this is a costly misconception. MCP is not just a technical detail; it’s a platform strategy that determines how quickly your organization can deploy reliable AI solutions, manage regulated data, and collaborate with CROs and partners. By standardizing the “connection layer” between AI models and the real world, MCP makes integrations repeatable, discoverable, and scalable. Changes in this foundational layer ripple across the organization, influencing everything from data workflows and regulatory compliance to patient engagement strategies.
What MCP Actually Is (in simple)
Think “USB-C for AI.” MCP defines a common way for AI agents (clients) to talk to data and services (servers) so developers don’t build bespoke adapters for every system. That reduces integration overhead and makes it easier to swap or scale AI components.
Two basic pieces: MCP servers expose data or tools (a knowledge base, lab instrument, clinical system, etc.); MCP clients (AI agents) discover and call those servers to get context or perform actions. The result is a standardized, bidirectional connector pattern.
Evidence of Rapid Adoption — Why This Is Not Hypothetical
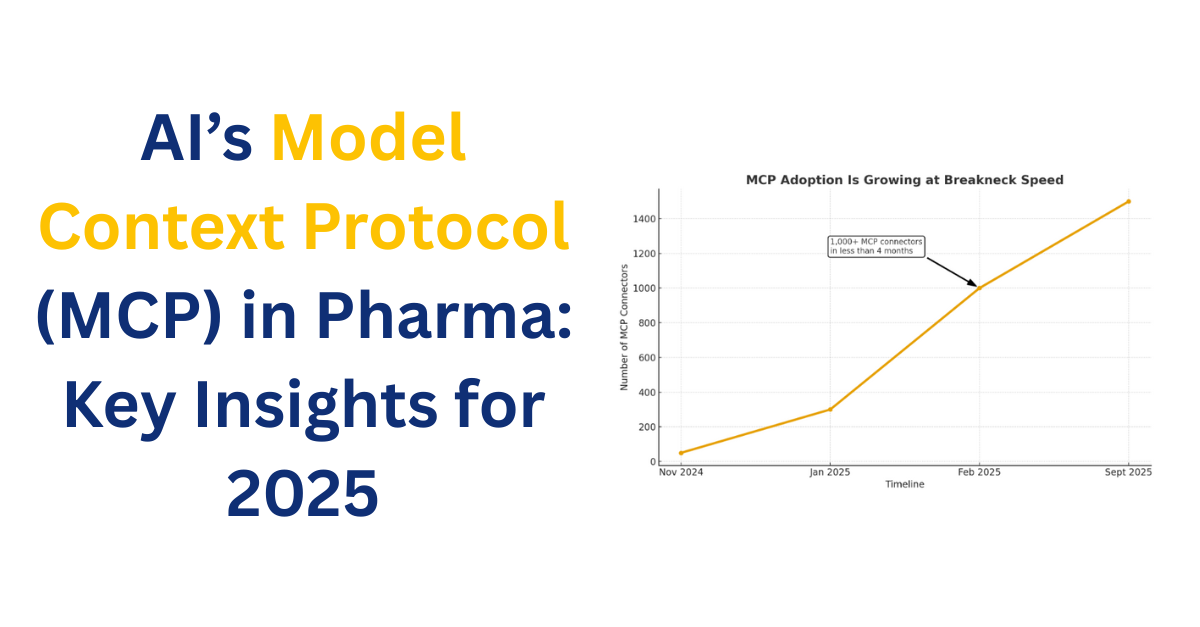
The protocol moved from announcement to ecosystem in months: by early 2025, community developers published hundreds, and reporting indicates 1,000+ community MCP connectors existed within a few months of launch. Major platforms and companies are experimenting with MCP servers and clients, and some OS ecosystems are exploring first-class support for MCP integrations. That rapid pace means MCP will influence product roadmaps and procurement choices sooner than many leaders expect.
Here’s the growth trend chart showing MCP adoption from Nov 2024 to Sept 2025, with the milestone callout at Feb 2025.
Why MCP Matters for Pharma Data Strategy — Five Tangible Business Outcomes
1) Break Down the Single-Source-of-Truth Problem Without Massive Rewrites
Many pharma companies still struggle with fragmented systems: LIMS, eTMF, EDC, regulatory repositories, CRO portals, and R&D notebooks live in different formats and places. MCP doesn’t magically normalize every schema, but it does let AI agents query those sources in place, apply transformations or sanitizations, and synthesize answers. That reduces the need for costly ETL rewrites when you want to power new AI features. The practical outcome: faster time to production for AI capabilities that require cross-system context.
2) Make Clinical-Trial Analytics and Design More Iterative
Designing trials is data-hungry and iterative. With MCP, AI agents can connect to historical trial datasets, real-world evidence sources, and protocol registries to simulate inclusion/exclusion scenarios, estimate enrollment timelines, and flag potential safety signals. Because MCP connectors can be reused across models, iterative experiments (e.g., try design A vs. B) become operationally feasible without rebuilding integrations each time. This can shorten protocol cycles and reduce the cost of pre-study feasibility analysis.
3) Improve Regulatory Intelligence and Submission Readiness
Regulatory landscapes evolve fast. An MCP-enabled agent can pull content from regulatory databases, internal change logs, and labeling repositories to produce alerts and draft submissions. When changes are detected, the same connector stack can automate evidence gathering and produce audit trails that are useful during inspections, if governance and validation are properly designed. Atlas Compliance complements this by providing real-time FDA enforcement data, inspection histories, and a large database of Form 483s and warning letters, helping teams stay audit-ready and aligned with evolving regulatory expectations.
4) Bring Pharmacovigilance and Safety Monitoring Closer to Real Time
Safety teams face enormous data heterogeneity: adverse event reports, EMR extracts, literature, and social chatter. MCP architectures let specialized AI agents access each relevant feed without copying or reshaping every dataset into a single warehouse. For safety surveillance, this means faster signal detection, automated triage, and prioritized case processing, again, provided validation and auditability are baked in.
5) Create New Customer and Partner Experiences
Imagine a CRO providing an MCP endpoint that surfaces study status, monitoring logs, and lab metrics to clients’ AI assistants. Clients could build their own models that periodically poll the CRO’s MCP server and generate stakeholder reports. That turns manual reporting into automated, near-real-time collaboration tools and becomes a commercial differentiator for service providers.
(Each of the above is not theoretical; vendor and community writeups show prototypes doing these sorts of tasks in non-regulated industries, and early life-science experiments are appearing.)
Numbers & the Current Landscape (What the Data Shows)
- Ecosystem growth: Community projects reporting more than 1,000 MCP servers/connectors in early 2025 is a practical signal of developer momentum. Rapid connector growth reduces the marginal cost of trying MCP in pilots.
- Platform buy-in: Major platform vendors and OS actors have signaled interest or early support for MCP; this accelerates enterprise readiness. Wide platform support makes MCP less “experimental” for regulated businesses.
- Security reporting: Early warnings in 2025 about prompt injection, credential leakage, and overbroad permission scopes highlight that protocol adoption without controls increases exposure. Expect more tooling and compliance frameworks to appear in the next 12–18 months.
The Risk Profile: MCP Creates New Failure Modes You Must Plan For
MCP makes integrations easier, and the easier it is, the greater the risk of accidental overexposure. Key threat vectors:
- Token and credential theft: If an MCP server stores OAuth tokens, compromise can give broad access. Attackers can spin up malicious MCP clients that query data at scale.
- Prompt injection & data exfiltration: Agents that execute actions need strict instruction controls; otherwise, crafted inputs can trick agents into leaking or misusing data.
- Excessive scope of access: Early community servers often requested broad permissions for convenience. Production deployments must utilize least-privilege scopes and obtain explicit consent.
- Auditability / validation gaps: For regulated submissions, the chain of custody for MCP-mediated reports must be demonstrable. That requires strong logging, versioned outputs, and documented validation of AI components.
Bottom line: MCP reduces integration friction, but it also amplifies blast radius if governance is lax.
Governance & Security Playbook (Practical Steps for Leaders)
- Map high-value connectors first: Identify 3–5 data sources that could provide the biggest business lift (e.g., trial status dashboards, eTMF index, safety case database). Prioritize connectors with clear ROI.
- Adopt least-privilege and token rotation: Ensure MCP servers request the minimum necessary scopes and rotate credentials. Use ephemeral tokens where possible and place connectors inside hardened service accounts.
- Policy enforcement & runtime checks: Invest in an MCP-aware policy engine that can enforce redaction, rate limits, and forbidden operations at runtime.
- Audit trails for validation: Every AI interaction that contributes to a regulated decision should record inputs, model versions, connector identity, and outputs. Keep immutable logs for inspections and RCA.
- Human-in-the-loop for high-risk flows: For regulatory documents, clinical decisions, or labeling changes, require human review and a documented approval workflow.
- Vendor & third-party review: Treat MCP endpoints offered by partners as a 3rd-party risk: require documentation, pen tests, and SLAs that include security incident response.
Validation, Compliance, and Audit Readiness — What Changes with MCP
Regulators don’t regulate protocols; they regulate outcomes: data integrity, traceability, and patient safety. When MCP is part of your stack, auditors will ask for evidence that:
- Data accessed via MCP is accurate and authorized.
- AI outputs used in decision-making are reproducible and traceable.
- Access controls and segregation of duties are enforced.
- Validation evidence exists for automated transformations and scripts used by MCP agents.
This means validation strategies must expand beyond “system qualification” to include connector qualification and AI component governance. Teams should document connector design, permission scopes, test harnesses that demonstrate correct behavior, and a remediation plan for connector failures.
Realistic Timelines & ROI Expectations
MCP lowers the upfront engineering cost of integrations, but business value still requires domain work: defining data semantics, building labeled datasets, validating AI outputs, and integrating outputs into operational workflows. Typical pilot cadence:
- 0–3 months: Identify use case, build a safe MCP server wrapper for a single non-critical data source.
- 3–9 months: Iterate with models, add governance controls, and demonstrate measurable KPIs.
- 9–18 months: Expand connectors, harden security, and move to regulated workloads after validation.
Key strategic payoff: reusability. One validated MCP connector can power many AI use cases, amplifying ROI as more agents are adopted.
Use-Case Snapshots (Concise Examples)
- Study status API for sponsors: Expose non-PII study metadata via an MCP server. Sponsors build lightweight agents to produce weekly status reports.
- Automated literature trackers: MCP connector surfaces PubMed updates + internal competitor reports to a research assistant agent for rapid brief generation.
- LIMS query agent (read-only pilot): Allow an AI agent to query instrument run summaries and flag anomalies to lab staff; manual confirmation required.
- Safety triage assistant: MCP pulls structured adverse event feeds and literature references; AI ranks cases for human review.
Technology & Vendor Landscape
- Anthropic: Launched MCP and published reference implementations; framed MCP as an open standard for secure two-way data access.
- Community projects & developer tutorials: Show many connectors available for platforms like GitHub, Notion, Slack, and cloud storage.
- Platform & security vendors: Early guidance and controls signal enterprise readiness and attention to risk.
When to Move Fast vs. Move Carefully
- Move fast (pilot now): Clear, non-PII use cases with measurable operational KPIs. Low regulatory risk but high time savings.
- Move cautiously: Connector touches PHI, GxP-controlled records, or affects patient safety. Require stronger validation and staged rollout with human oversight.
- Partner & vendor caution: Require security documentation and contractual commitments on data handling.
Future Expectations (Next 12–36 Months)
- Tooling & registries: Centralized MCP registries, connector marketplaces, and signed connector catalogs for discovery and trust.
- Policy & runtime controls: Security vendors shipping MCP-aware enforcement engines.
- Healthcare adaptations: Variants or extensions (e.g., HMCP) align MCP with HIPAA and Part 11.
- Standards & best practices: Industry associations will publish implementation and validation guides.
- Composability: Protocol enables the assembly of AI agents combining multiple models and connectors.
Executive Checklist — 10 Actions to Get Started
- Convene a cross-functional MCP working group (IT, Security, Clinical Ops, Regulatory, Legal).
- Identify 2 high-impact, low-risk pilot use cases.
- Require least-privilege tokens and ephemeral credentials.
- Build audit logging and immutable trails.
- Define validation requirements upfront.
- Engage vendors about the MCP roadmap and security posture.
- Budget for an MCP policy engine or runtime control.
- Draft internal policy for connector publishing and consumption.
- Train staff on workflows and human-in-the-loop controls.
- Consider hiring a fractional Chief AI Officer to drive adoption and governance.
Conclusion — Strategic Framing for the C-Suite
MCP isn’t merely a developer convenience — it’s an architectural inflection point. For life sciences, it changes how you think about data access, validation, vendor relationships, and the speed at which AI can deliver clinical and commercial value. The promise is real: faster experiments, more reusable connectors, and new customer experiences. The risk is real, too: broader attack surfaces, governance gaps, and validation requirements that could trip up regulated workloads.
Treat MCP as a strategic capability: pilot fast where risk is low, invest in governance and validation where risk is high, and build organizational muscle, people, process, and tooling that turns technical standards into a durable business advantage.
Most Frequently Asked Questions
Q1 — Is MCP secure enough for PHI and regulated systems today?
Short answer: not by default. MCP implementations require strict governance, least-privilege tokens, and validated connectors to be safe for PHI and regulated use; consider healthcare-specific extensions and vendor assurances.
Q2 — Do I need to replatform my data warehouse to use MCP?
No. MCP can expose data in place; duplication isn’t required. Connectors still need validation and semantic clarity.
Q3 — Will MCP replace APIs and existing integrations?
Not immediately. MCP reduces the need for bespoke connectors, but existing APIs will coexist. Over time, MCP may be favored for discoverability and reuse.
Q4 — What’s a sensible first pilot for pharma?
A read-only study status or knowledge-synthesis pilot: limited scope, measurable KPIs, and no direct patient safety impact.
Q5 — How do we prove regulatorily that an MCP-enabled AI output is valid?
Keep immutable logs, version all models and connectors, document test cases and acceptance criteria, and require human sign-off for regulated outputs. Include negative test cases for connector failures and injection attempts.