Have you thought about the real cost of poor quality in your business? In 2023, the U.S. pharmaceutical industry faced 301 drug shortages per quarter, a 13% increase from the previous year. This not only disrupts the supply chain but also puts patients at risk of not getting the medications they need. On top of that, the price of new drugs rose by 35% last year, reaching an average of $300,000 per drug. This adds strain on healthcare systems and patient affordability.
In manufacturing, the picture isn’t much better. 90%of clinical drug development projects fail due to poor quality control early in the process. These failures cost companies millions and delay important products from reaching the market.
These numbers highlight that quality control is a serious risk to your business’s finances, compliance, and reputation.
In this blog, we’ll explore what quality control really means, the methods you can use to improve it, and how taking a proactive approach can protect your business from costly mistakes and regulatory issues.
To understand how these issues can be avoided, we first need to define what quality control truly is and why it matters across various industries.
Key Takeaways
- Quality control (QC) ensures products meet safety, compliance, and quality standards across industries.
- Key QC methods include Statistical Process Control (SPC), end product testing, sampling, and process validation.
- Industries like pharmaceuticals, food production, and manufacturing apply QC to maintain safety and avoid costly defects.
- Effective QC boosts operational efficiency and customer satisfaction while reducing risks and regulatory penalties.
- Common QC challenges include documentation issues, supply chain management, and adapting to evolving regulations.
What is Quality Control?
Quality control (QC) refers to the processes and procedures used to ensure that products meet specific quality standards. It involves inspections, tests, and ongoing monitoring of production to identify and correct issues early on. QC is essential for delivering products that are safe, reliable, and compliant with regulations.
In regulated industries like pharmaceuticals and food production, quality control is even more critical. Failure to meet quality standards can result in recalls, fines, or legal consequences. While the concept of QC is universal, its application can differ depending on industry requirements and regulatory frameworks.
By exploring how quality control works, we can better understand its methods and why it’s so critical in ensuring product consistency. Let’s look at some of the most commonly used quality control methods.
Quality Control Methods
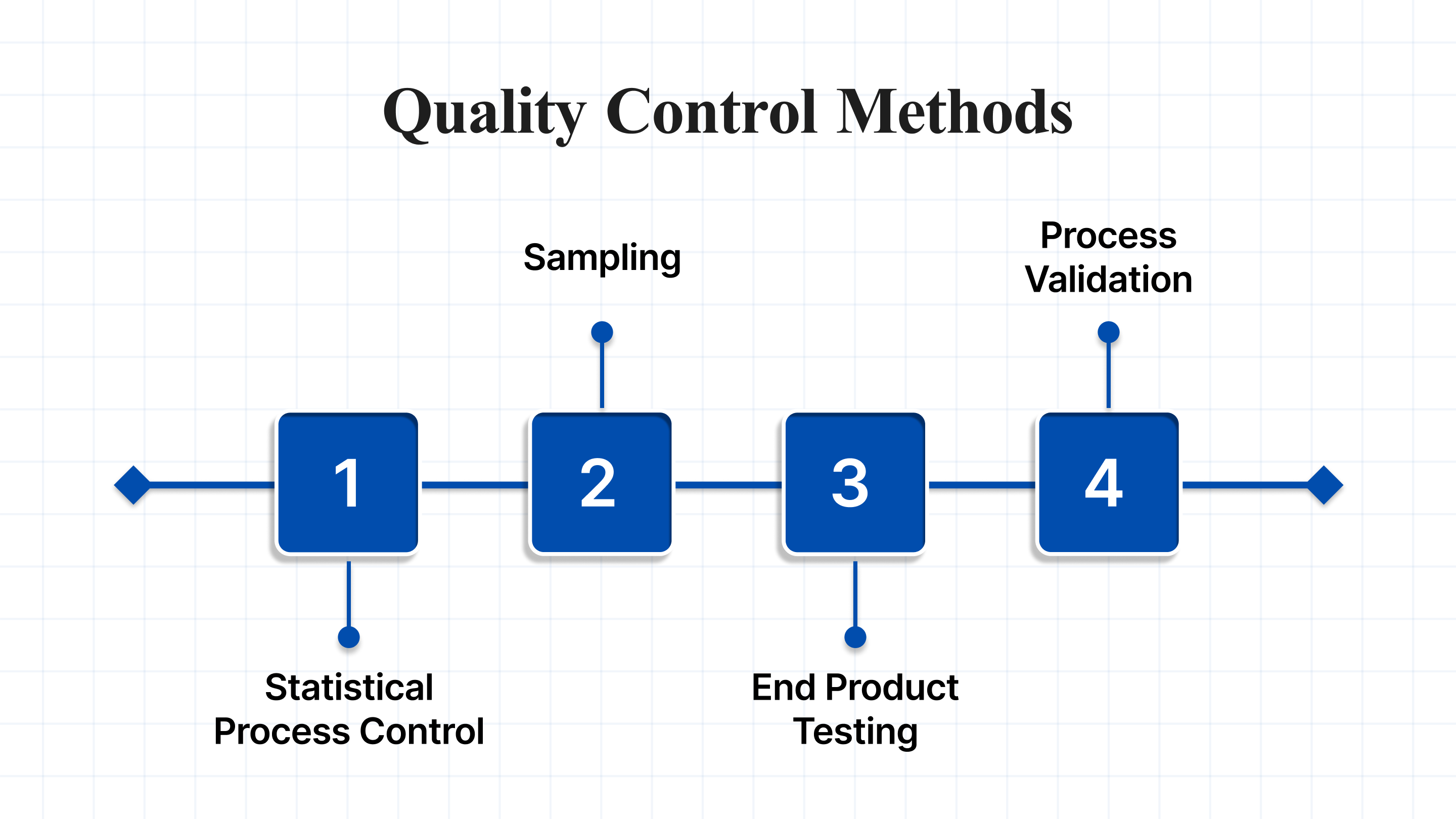
There are several methods used in quality control, each designed to detect problems early and prevent defects from reaching customers. Here are some of the most common approaches:
- Statistical Process Control (SPC): This method uses statistical data to monitor and control processes, helping ensure they stay within acceptable limits.
- Sampling: This involves selecting random samples from a batch and testing them for quality, offering an efficient way to assess the overall quality without testing every product.
- End Product Testing: The final products are tested to ensure they meet the required standards before they are released to consumers.
- Process Validation: This ensures that manufacturing processes are capable of consistently producing products that meet the desired specifications.
These methods are key to maintaining consistent quality across industries. Understanding how they work can provide valuable insight into their real-world applications. Next, let’s explore how quality control looks in practice across various sectors.
Types of Quality Control by Industry
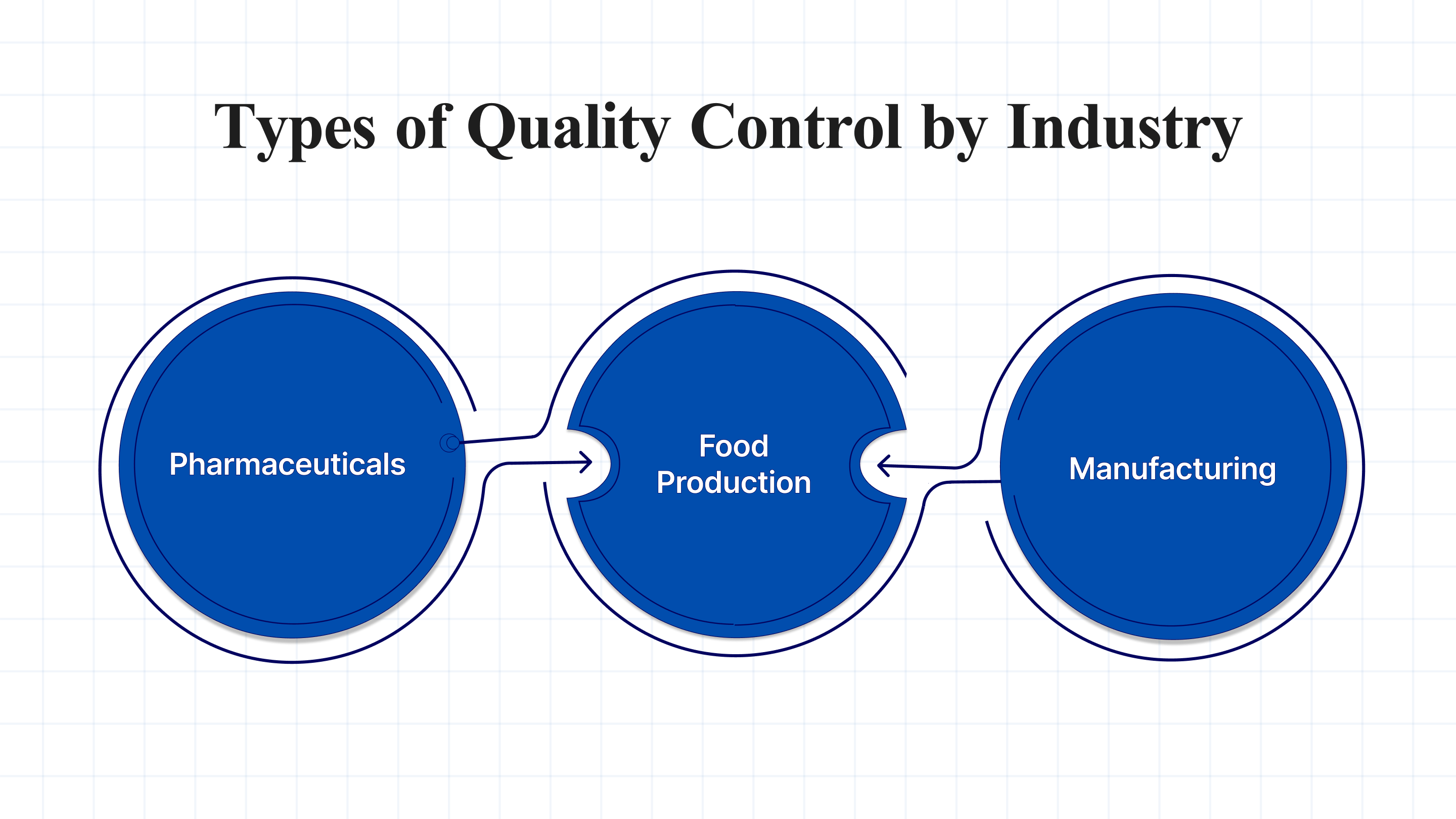
Quality control methods vary slightly depending on the industry, but they all share the same goal: ensuring product safety, compliance, and consistency. Here’s how QC is applied across different sectors:
- Pharmaceuticals: QC in pharma involves stringent testing and validation to ensure drugs are safe and effective. This includes batch testing, validating production processes (such as GMP), and ensuring the absence of contaminants.
- Food Production: In the food industry, QC focuses on monitoring raw materials, ensuring cleanliness, and testing finished products to comply with safety standards.
- Manufacturing: In manufacturing, SPC and regular product inspections help identify defects early and reduce the risk of defective products reaching customers.
Each sector faces unique challenges and regulations, but the principles of quality control remain the same. With a solid understanding of QC methods, it’s clear why maintaining strong quality control processes is essential. This brings us to why quality control matters so much.
Why Is Quality Control Important?
Quality control plays a critical role in ensuring the safety, compliance, and satisfaction of customers. The benefits of QC include:
- Ensuring Safety and Compliance: QC ensures that products meet safety standards and comply with regulations, reducing the risk of harmful products reaching consumers.
- Boosting Operational Efficiency: By identifying problems early, businesses can avoid costly recalls, production delays, and inefficiencies.
- Improving Customer Satisfaction: Delivering consistently high-quality products builds trust with consumers, leading to repeat business and positive recommendations.
When QC is properly managed, it can have a positive impact on every area of your business. But even with these benefits, many businesses still struggle to maintain effective quality control. Let’s take a look at some common challenges businesses face and how to overcome them.
Common Quality Control Challenges and How to Overcome Them

Quality control may seem straightforward, but businesses often face challenges in maintaining effective QC systems. Some of the most common issues include:
- Documentation and Record-Keeping: Inconsistent or incomplete documentation can lead to compliance issues and costly mistakes.
- Managing Supply Chain Quality: Ensuring that suppliers meet quality standards is a challenge, especially when sourcing materials globally.
- Evolving Regulations: Keeping up with changing regulations, especially in industries like pharmaceuticals, can be difficult.
Fortunately, these challenges can be addressed with the right strategies. By using tools like Atlas Compliance, businesses can proactively track quality control issues as they happen and anticipate potential problems before they occur, helping to maintain compliance and efficiency. With the right approach, businesses can streamline their quality control processes and improve compliance. Now, let’s discuss how Atlas Compliance can support you in staying proactive with quality control.
Conclusion
Quality control is more than just a process; it’s a critical element for ensuring compliance, reducing risk, and maintaining a strong brand reputation. By implementing effective QC methods and overcoming common challenges, businesses can enhance efficiency and deliver products that consistently meet safety and quality standards.
Atlas Compliance provides tools and insights that enable businesses to stay proactive with quality control, facilitating easier management of inspections and maintaining inspection readiness.
Contact Atlas Compliance today to learn how our platform can help you improve your quality control processes and stay ahead of regulatory risks.
FAQs
QC focuses on identifying defects, while QA works to prevent them by improving systems and processes.
SPC uses statistical methods to detect variations in processes early, preventing defects from becoming a larger problem.
It ensures that manufacturing processes consistently produce products that meet quality standards.
These include poor documentation, inconsistent supply chain quality, and adapting to changing regulations.
Atlas provides real-time insights and predictive analytics to help businesses stay ahead of quality control issues and ensure compliance.