What happens when a batch fails to meet the required specifications during production? In regulated industries like pharmaceuticals, biotechnology, and life sciences, this scenario is not just a minor issue; it’s critical. Out-of-specification (OOS) results can disrupt production, delay launches, and even lead to regulatory action. In 2023, the pharmaceutical industry faced significant drug shortages, with 309 ongoing drug shortages reported by the American Society of Health-System Pharmacists (ASHP) by mid-year. This marked the highest number of shortages in nearly a decade.
Understanding OOS, how it’s investigated, and how to prevent its recurrence is key to maintaining product quality and meeting regulatory compliance. In this blog, we’ll break down the core concepts of OOS, the investigation process, its regulatory implications, and best practices for managing OOS results effectively.
Now that we’ve set the stage, let’s first understand what OOS really means and why it’s such an important concept in regulated industries.
Key Takeaways
- OOS (Out of Specification) results indicate product non-compliance in regulated industries.
- Regulated sectors like pharmaceuticals and food must adhere to strict OOS handling protocols.
- OOS results can lead to recalls, legal issues, and fines if not managed properly.
- Root causes of OOS may include testing errors, equipment malfunction, or material inconsistencies.
- Effective OOS management requires robust quality control, clear procedures, and preventive strategies.
What is OOS?
Out of Specification (OOS) refers to a test result that falls outside the established acceptance criteria defined by regulatory bodies, manufacturers, or official standards. It applies to any test, whether on raw materials, in-process materials, or finished products, that fails to meet the predefined standards. In the pharmaceutical industry, OOS results are critical indicators that signal potential problems in the production process.
Regulatory bodies like the FDA require manufacturers to thoroughly investigate any OOS result to determine its cause and ensure the product’s quality and safety. For instance, OOS results could be related to potency, purity, or other specifications outlined by the FDA or compendia such as the USP (United States Pharmacopeia).
With a clear understanding of OOS, it’s essential to know the common causes behind these results. Identifying the root cause is crucial for preventing its recurrence.
Common Causes of OOS Results
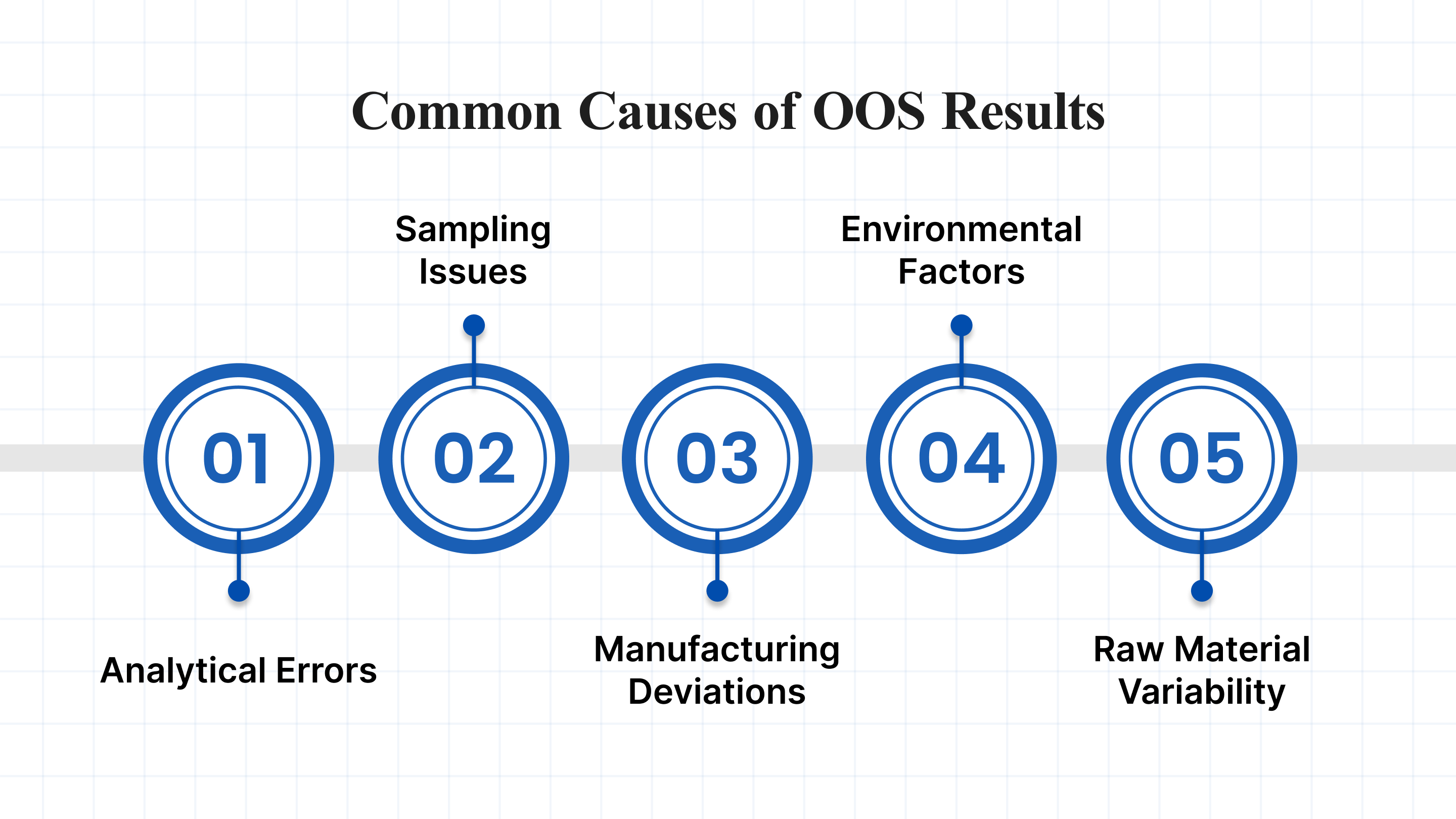
OOS results can stem from various factors during manufacturing, testing, or handling. Some of the most common causes include:
- Analytical Errors: Testing errors, such as equipment malfunction, improper calibration, or human error, can lead to inaccurate results. A thorough investigation helps identify if the OOS result was caused by a laboratory mistake.
- Sampling Issues: Incorrect sampling procedures or mishandling of samples can lead to erroneous test results. Ensuring proper sampling techniques is essential in preventing such issues.
- Manufacturing Deviations: Variations in the production process, such as incorrect mixing times, temperature fluctuations, or faulty equipment, can affect the quality of the product.
- Environmental Factors: Factors such as humidity, temperature, or contamination can affect the integrity of raw materials and final products.
- Raw Material Variability: Inconsistent or low-quality raw materials can lead to deviations from product specifications, resulting in OOS results.
Once we understand the common causes, we can look into the investigation process, which helps identify the root cause and prevent similar issues in the future.
Investigation Process for OOS Results
When an OOS result occurs, a structured investigation must take place to determine the cause. The investigation typically happens in two phases:
Phase I: Laboratory Investigation
The first step is to investigate whether the OOS result is a result of laboratory error. The following steps are often taken:
- Review Test Procedures: Check if the procedures followed during testing were in line with the Standard Operating Procedures (SOPs).
- Instrument Calibration: Ensure all instruments were properly calibrated and functioning.
- Analyst Review: Assess whether the analyst followed the correct testing methods.
If errors are found during this phase, a retest may be conducted, and the issue may be resolved. However, if no error is detected, the investigation proceeds to the next phase: a more extensive review of the entire production process.
Phase II: Full-Scale Investigation
If the laboratory phase does not yield a cause, a broader investigation is initiated, which includes:
- Reviewing Production Records: Examine batch records, including manufacturing and quality control logs, to identify any deviations from the established production process.
- Material Inspection: Investigate raw materials used in production to ensure they meet specifications.
- Equipment Review: Inspect any equipment used in the production or testing processes to ensure it operated within the required parameters.
By conducting a thorough investigation, businesses can identify if the OOS result was an isolated event or indicative of a larger systemic issue. This leads to the implementation of corrective and preventive actions (CAPA), a critical step in maintaining quality control. Once the investigation process is understood, we can then explore the regulatory implications of OOS results.
Regulatory Implications
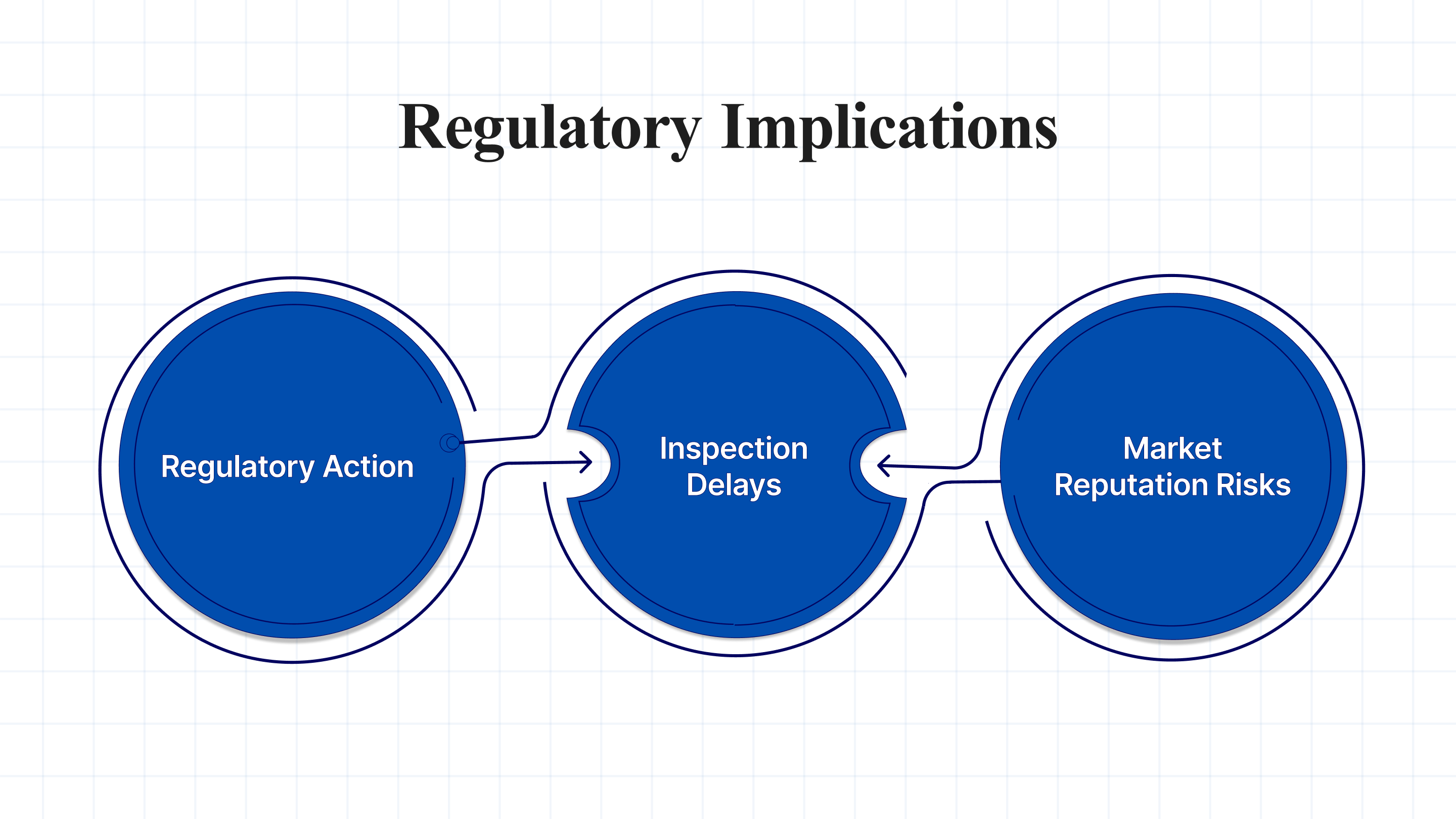
OOS results have significant regulatory implications. The FDA and other regulatory bodies mandate that any OOS result must be fully investigated and documented. If an OOS result is not addressed adequately, it can lead to:
- Regulatory Action: This includes warning letters, fines, or more severe measures like product recalls.
- Inspection Delays: Inadequate OOS handling can raise red flags during regulatory inspections, delaying approval or resulting in penalties.
- Market Reputation Risks: A failure to address OOS results and potential non-compliance can damage a company’s reputation, affecting customer trust and brand value.
Manufacturers must maintain detailed records of the OOS investigation, corrective actions taken, and any preventive measures implemented. This documentation is crucial for demonstrating compliance during inspections and audits. With a clear understanding of the regulatory requirements, businesses should adopt best practices for managing OOS results to minimize risk.
Best Practices for Managing OOS Results
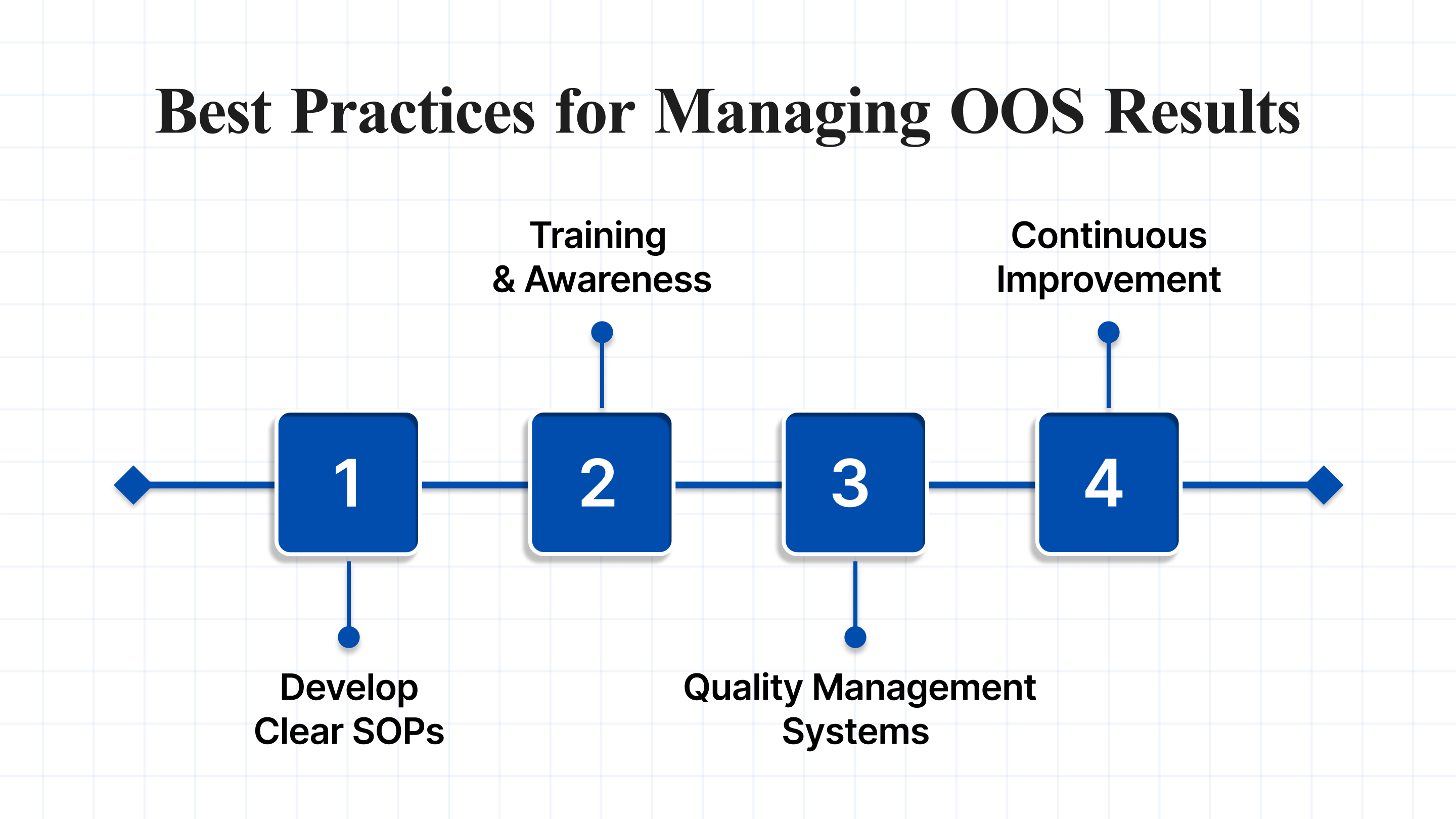
To prevent OOS results from becoming recurring issues, businesses should follow best practices for effective management:
- Develop Clear SOPs: Ensure that there are well-defined Standard Operating Procedures (SOPs) for handling OOS results. These SOPs should include steps for initial testing, investigation, documentation, and corrective actions.
- Training and Awareness: Regularly train staff on OOS procedures and the importance of identifying and addressing any discrepancies in product testing or production.
- Use Quality Management Systems (QMS): Implement a robust QMS that monitors and manages OOS occurrences across the production process. A strong QMS can help streamline OOS investigations and ensure compliance.
- Continuous Improvement: Use data from OOS results to identify trends and areas for process improvement. This helps in refining manufacturing processes and reducing the likelihood of future OOS results.
Conclusion
Out-of-specification (OOS) results are an inevitable part of manufacturing, but understanding their causes and having an effective investigation and management process in place can help minimize their impact. By investigating OOS results thoroughly, adhering to regulatory guidelines, and continuously improving processes, businesses can maintain product quality, ensure compliance, and avoid costly regulatory issues.
OOS results are not just regulatory hurdles; they are opportunities to improve your processes and prevent future failures. By handling OOS results properly, businesses can turn potential risks into opportunities for better performance and product quality.
Atlas Compliance offers tools to help you stay ahead of OOS results with real-time insights, predictive analytics, and seamless integration with your existing quality management systems. With our platform, you can proactively manage OOS incidents, streamline investigations, and maintain inspection readiness.
Reach out to Atlas Compliance today and see how we can help you stay proactive in managing OOS and maintaining compliance.
FAQs
OOS refers to any test result that falls outside the established specifications set by regulatory bodies or manufacturers.
Causes include analytical errors, sampling issues, manufacturing deviations, environmental factors, and raw material variability.
OOS results are typically investigated in two phases: laboratory investigation and a broader investigation involving production records and equipment checks.
Failure to investigate and address OOS results can lead to regulatory actions such as fines, product recalls, or inspection delays.
Through clear SOPs, staff training, a robust QMS, and continuous process improvement to prevent future OOS occurrences.