Changes in a project vary in nature. Some are unexpected, such as weather delays or illness, while others are formally requested. Regardless of how they arise, each change must be carefully evaluated and either accepted or rejected. Once a change is approved, it initiates a structured change control process to help ensure the project stays on track and within budget.
In this blog, you’ll explore the fundamentals of change control and learn how modern tools can help streamline your processes and improve compliance efforts.
TL;DR:
- Change control is a structured process to evaluate and manage project changes, ensuring alignment with project goals.
- It reduces risks, maintains quality, controls costs, and ensures regulatory compliance, especially in regulated industries.
- The process involves 6 key steps: submitting requests, assessing impact, evaluating readiness, decision making, planning execution, and closing changes.
- An example in pharmaceutical manufacturing illustrates the practical application of change control.
- AI-powered tools can streamline change control and compliance management.
What is Change Control?
The change control process is a structured approach to managing project changes. Its purpose is to ensure that any modifications are thoughtfully considered, supporting the project’s goals rather than disrupting them, and that all necessary resources are in place for successful implementation.
Typical situations that require the use of the change control process include:
- Stakeholder feedback, where user input indicates the current direction doesn’t meet their needs.
- Changes to project scope, such as adding new features.
- Regulatory requirements, when updates are needed to comply with legal or industry standards.
- Resource shifts, like reallocating team members or budget to different tasks.
- Timeline revisions, involving changes to deadlines or scheduling adjustments.
Types of Change Control
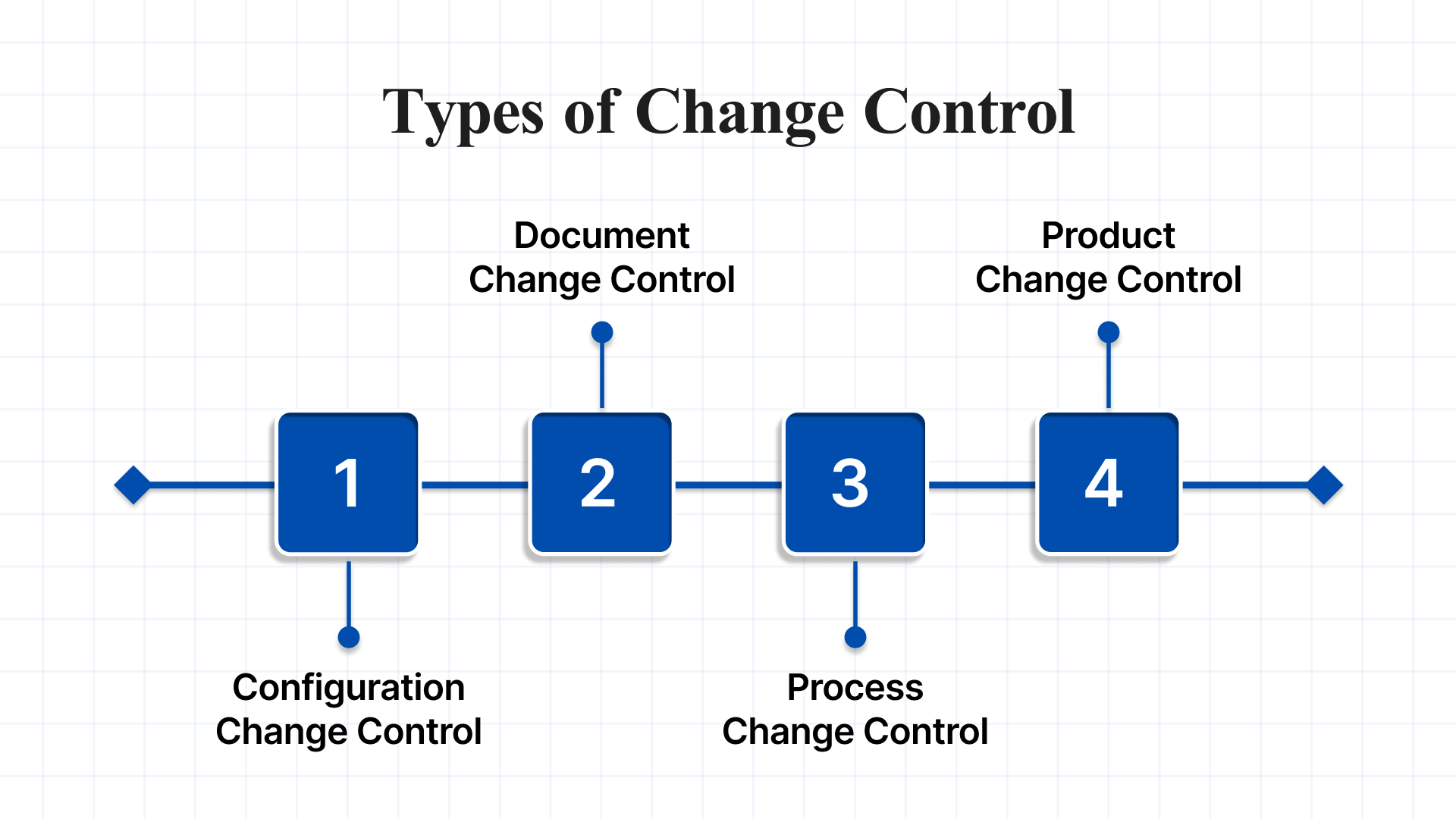
To manage changes effectively, it’s important to understand the different types of change control, each addressing a specific domain of project modifications. There are four primary categories:
- Configuration Change Control: This manages changes to systems and technical configurations, such as IT infrastructure, hardware, software, or networks. It ensures updates are controlled, logged, and compatible with existing setups.
- Document Change Control: This focuses on managing revisions to formal documents, including SOPs, technical manuals, policies, and regulatory submissions. It ensures consistency, version control, and traceability.
- Process Change Control: This type oversees adjustments to operational or business processes. It ensures that workflow or procedural changes are properly assessed and implemented without introducing unnecessary disruption.
- Product Change Control: This governs alterations to product design, specifications, or components, particularly in manufacturing, the life sciences, and engineering. It ensures the product continues to meet quality and regulatory requirements.
Change Control vs. Change Management
It’s easy to mix up change control and change management. They’re related, but not the same. Think of change control as one component within the broader scope of a change management strategy.
- Change Control: Change control refers to the structured process for handling modifications within a project. It ensures that all proposed changes are clearly documented, evaluated, and either approved or declined based on impact. A strong change control process defines success criteria, streamlines workflows, facilitates team communication, and lays the groundwork for long-term project success.
- Change Management: Change management, on the other hand, is the comprehensive approach used to prepare, support, and guide teams through change. It includes managing budgets, timelines, communication plans, and resource allocation. While change control focuses on individual change requests and their approval process, change management encompasses the full strategy for implementing and sustaining change across an organization.
Why is Change Control Important?
Change control is essential for maintaining consistency, managing risks, ensuring compliance, and maintaining quality in regulated industries through thorough evaluation and effective management of changes. Here’s why:
- Risk Reduction: Thorough evaluation of changes helps prevent safety and compliance risks.
- Project Control: Keeps projects on time and within scope by managing changes effectively.
- Regulatory Compliance: Ensures all changes meet industry standards like FDA requirements.
- Quality Assurance: Maintains product quality by controlling how and when changes are made.
What Are the Benefits of a Well-Executed Change Control?
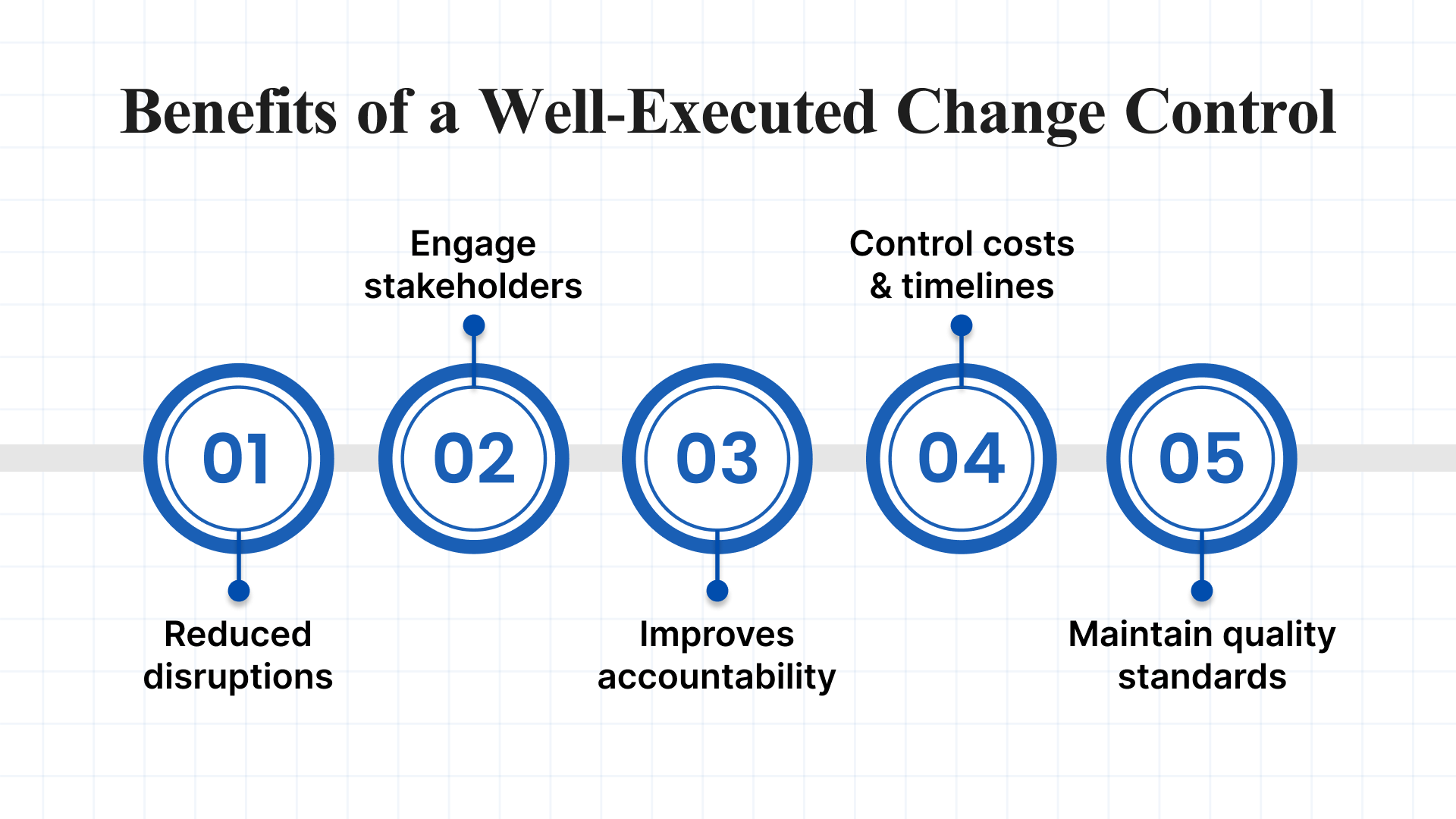
Establishing a change control process brings structure to your team by improving coordination through organizational tools and enhancing efficiency around deliverables and deadlines. It also plays a key role in minimizing the risks associated with poorly managed changes. A well-executed change control process offers several key benefits:
- Reduced disruptions: A structured approach to managing changes helps keep your project on track. By anticipating and addressing potential risks, you’re better equipped to maintain momentum.
- Engage stakeholders: A defined change control process provides a transparent channel for stakeholder input and ensures you follow up with them after decisions are made. This fosters collaboration and shared commitment to project success.
- Improves accountability: Recording each change, who approved it, and the rationale behind it promotes transparency and reinforces team accountability.
- Control costs and timelines: Evaluating changes thoroughly allows you to stay aligned with your budget and deadlines, reducing the risk of delays and overspending.
- Maintain quality standards: Reviewing proposed changes against your quality benchmarks helps ensure that nothing is accepted that could compromise the final outcome, resulting in a stronger, more reliable deliverable.
6 Steps to the Change Control Process

Understanding and following each of these steps helps ensure changes are properly managed and deliver value without disrupting your project goals. Along the way, keep your change log and change request forms up to date to maintain visibility and control.
1. Submit a Change Request
Changes can be suggested by anyone involved in the project, including team members, stakeholders, or customers. To formally propose a change, complete a change request form that outlines the nature of the change and its potential benefits. Once submitted, the request is logged in your change log to begin the evaluation process.
2. Assess the Change Impact
Project managers and decision-makers assess the proposed change in the context of project scope, cost, timeline, risk, and resource availability. The goal is to weigh the benefits against potential disruptions. Key questions to ask include:
- Will the change delay the project?
- Are additional resources required?
- Does the change introduce new risks?
- How will other projects or teams be affected?
3. Evaluate Change Readiness
Before implementing any approved change, assess the organization’s ability to adopt it successfully. Consider factors such as strategic alignment, available capacity, team readiness, and potential resistance to ensure a successful implementation. If readiness is low, the risk of failure increases, even if the change itself is beneficial.
4. Make a Decision
The project manager presents the analysis and recommendations, but final approval lies with designated authorities (e.g., a change control board). Possible decisions include:
- Approve the change
- Approve with conditions
- Reject the change
- Defer the decision for later consideration
5. Plan and Execute the Change
Once a change is approved, it must be carefully planned. Develop a detailed implementation strategy that includes roles, responsibilities, timelines, and contingency plans (such as rollback procedures). Stakeholder alignment is key before executing the plan.
6. Close the Changes
After implementation, confirm that the change meets its objectives and that the requester is satisfied. Complete and file all associated documentation, update the change log, and conduct a final review to identify lessons learned for future improvements.
Change Control Process Example: Pharmaceutical Manufacturing
Scenario: A pharmaceutical company is manufacturing a batch of oral tablets. Midway through the production cycle, the team identifies an opportunity to switch from a plastic to a more sustainable blister packaging material without compromising product stability.
1. Propose the Change: The packaging engineer submits a formal change request to use a biodegradable blister pack instead of the standard plastic one. The proposal outlines the environmental benefits, potential cost changes, and initial compatibility findings with the product.
2. Assess the Impact: The Quality Assurance (QA), Regulatory Affairs, and Production teams assess the proposed change. They evaluate whether the new packaging complies with FDA regulations, if it requires stability testing, whether it affects the shelf life, and if regulatory filings (e.g., a post-approval CBE-30 supplement) are needed.
3. Evaluate Readiness and Make a Decision: The Change Control Board (CCB) reviews all findings. Since the new material may require regulatory notification and additional testing, they assess whether the company has the resources and time to proceed. Once the benefits outweigh the risks and readiness is confirmed, the board approves the change with specific implementation conditions.
4. Implement the Change: The QA team coordinates validation runs using the new blister packs. Stability testing is initiated, and the Regulatory Affairs team prepares supplemental submissions for FDA approval. Once results are confirmed, full-scale rollout of the new packaging begins.
5. Close the Change: After successful implementation and FDA acknowledgment (if required), the change is formally closed. All documentation, including validation reports, risk assessments, and change logs, is archived. A follow-up review confirms there are no unexpected issues post-change.
How Atlas Compliance Can Help?
Atlas Compliance provides AI-powered tools that streamline the change control process, particularly when dealing with FDA regulations.
- AI-Powered Document Search: Advanced Natural Language Processing (NLP) tools streamline the search for key information across regulatory documents, saving time, reducing manual effort, and lowering the risk of oversight.
- Predictive Compliance Analytics: It uses machine learning to analyze past inspection data and forecast potential compliance risks, identifying patterns that indicate potential compliance risks, enabling your team to proactively address issues before they escalate.
- Supplier Risk Management: Effectively evaluate and monitor supplier compliance to ensure adherence to relevant regulations and standards. Atlas supports regulatory adherence across your supply chain by identifying and mitigating supplier-related risks.
- FDA Inspection Intelligence: Gain access to a comprehensive database of FDA inspection records, including Form 483s, warning letters, and CFR citations. This insight enables you to identify compliance risks early and strengthen your processes.
- Regulatory Surveillance: Stay up-to-date with real-time monitoring of regulatory developments and inspection trends. This ensures your organization remains aligned with the latest FDA requirements.
- All-in-One Compliance Platform: With Atlas Compliance, you can oversee inspections, track regulatory updates, and prepare for audits—all within a unified platform designed to simplify and streamline compliance.
Conclusion
Implementing a structured change control process is crucial for managing projects successfully, especially in industries where compliance and regulatory standards are paramount. By following the steps outlined above and utilizing tools likeAtlas Compliance, you can ensure that changes are managed effectively, risks are minimized, and your organization stays audit-ready.
Ready to streamline your compliance processes? ExploreAtlas Compliance to see how our AI-powered platform can support your regulatory needs. Book a Demo Today!
FAQs
1. What is change control in Quality Assurance (QA)?
Change control in QA refers to a structured process used to manage modifications to products, processes, or systems in a controlled manner. It helps ensure that all changes are assessed, approved, implemented, and documented without compromising product quality or regulatory compliance.
2. What does change control mean, and why is it important?
Change control is a formal approach to managing changes systematically. It ensures that changes are introduced in a planned, reviewed, and approved way to avoid risks, minimize disruptions, and maintain the integrity of systems or processes. It is especially vital in regulated industries and quality management systems.
3. What is the key principle behind change control?
The core principle of change control is proactive preparedness, not reactive response. It emphasizes planning for change by evaluating the need, aligning resources, and managing the transition smoothly, ensuring minimal disruption and maximum control over quality and compliance.
4. What are the main steps in a change control process?
A typical change control process follows these five essential steps:
- Initiate a Change Request: Formally document and justify the need for the change.
- Evaluate the Request: Assess the impact, risks, and feasibility of the change.
- Develop a Change Strategy: Define how the change will be implemented and managed.
- Implement the Change: Execute the approved changes under controlled conditions.
- Close the Change Request: Review and document the outcomes, ensuring completion and compliance.
5. What is the main objective of change control?
The primary goal of change control is to evaluate whether a proposed change offers more benefits than risks or costs. It helps ensure that all changes are justified, controlled, and do not negatively impact product quality, safety, or regulatory compliance.
