tl;dr: The Model Context Protocol (MCP) significantly reduces human error in clinical data management and regulatory reporting by standardizing data interactions, automating processing, enabling real-time validation, and creating detailed audit trails. Its adoption leads to faster, more accurate, and compliant operations, reducing manual errors by up to 90% and accelerating regulatory reporting by 50–70%. MCP’s integration with AI ensures better data integrity, audit readiness, and streamlined workflows, making it a vital tool for life sciences and pharmaceutical organizations aiming for efficient and error-free clinical trials.
The process flow of the Model Context Protocol (MCP) highlights how data moves from extraction to validation while minimizing human error. Each stage, extraction, mapping, feedback, and validation- ensures data accuracy, regulatory compliance, and operational efficiency.
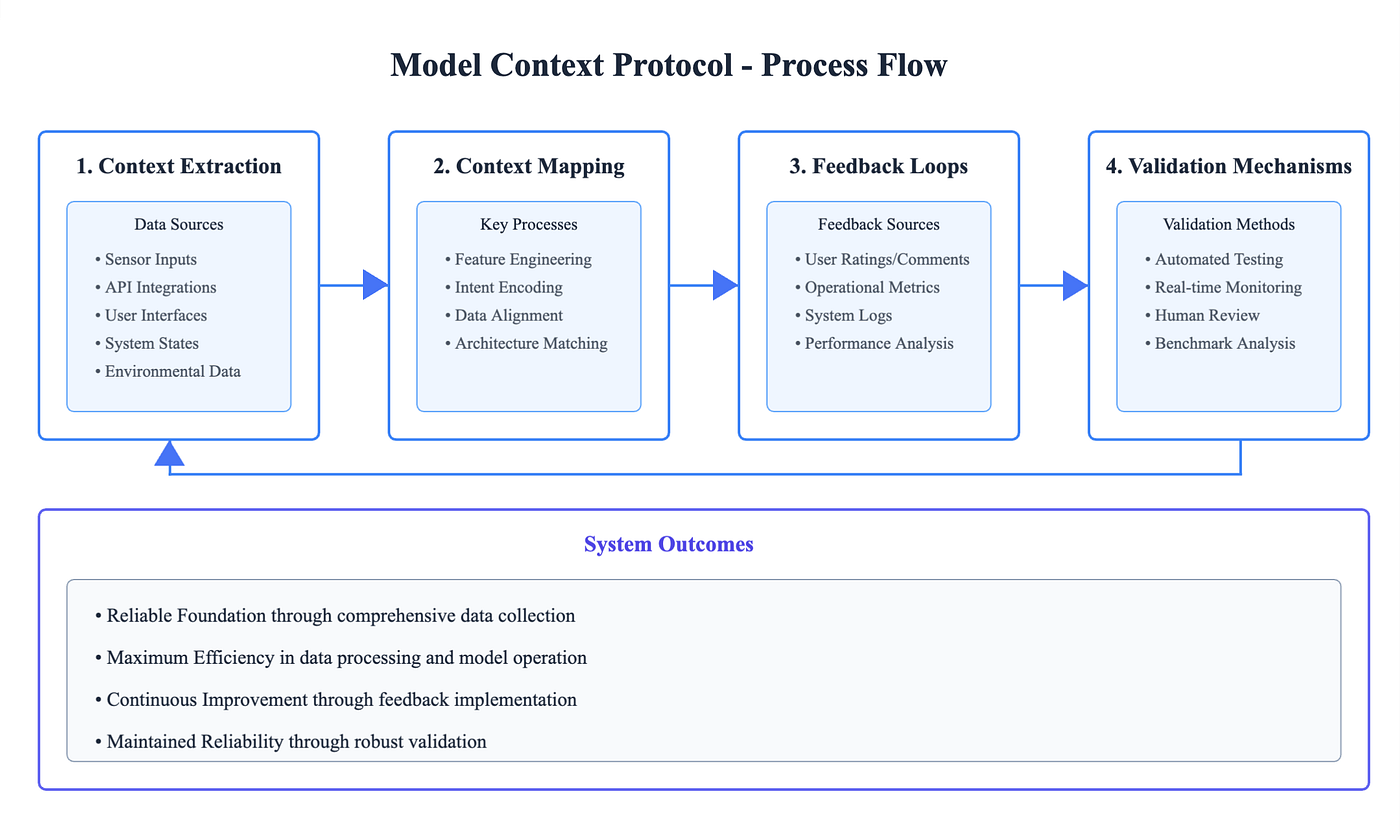
This flow illustrates how MCP systematically reduces manual intervention and human error in clinical data management and regulatory reporting. By standardizing inputs, aligning context, integrating feedback, and enforcing validation, MCP creates a cycle of continuous improvement, resulting in reliable data, faster reporting, and stronger compliance outcomes.
The Challenge of Human Error in Clinical Data Management
In the pharmaceutical and life sciences industries, clinical data management (CDM) and regulatory reporting are critical processes that ensure the integrity and compliance of clinical trials. However, these processes are often susceptible to human errors, which can lead to compromised data quality and regulatory non-compliance. The Model Context Protocol (MCP) has emerged as a transformative solution to mitigate these errors by providing a standardized framework for AI systems to interact with external data sources and services in a secure, controlled, and reproducible manner.
Understanding Human Error in Clinical Data Management
Human error in CDM manifests in various forms, including data entry mistakes, inconsistent data handling, and delayed reporting. Studies have shown that error rates in data processing methods can vary widely, with some methods exhibiting up to 2,784 errors per 10,000 fields. These errors not only compromise the quality of clinical data but also pose significant risks to patient safety and regulatory compliance.
The Role of MCP in Reducing Human Error
1. Standardization of Data Interactions
MCP provides a standardized framework for AI systems to access and interact with clinical data sources. This standardization ensures consistent data handling practices, reducing variability introduced by human intervention. By adhering to a uniform protocol, MCP minimizes the chances of errors arising from inconsistent data interactions.
2. Automation of Data Processing
Through the integration of MCP, AI systems can automate data queries and processing tasks, thereby reducing the need for manual data handling. This automation significantly decreases the likelihood of errors associated with manual data entry and processing. For instance, organizations implementing MCP have reported a 90% reduction in manual data entry errors.
3. Enhanced Traceability and Auditability
MCP facilitates the creation of detailed audit trails for data interactions, enhancing traceability. This feature ensures that all data modifications are logged and can be reviewed, promoting accountability and reducing the chances of undetected errors. Such traceability is crucial for compliance with regulatory standards like 21 CFR Part 11, which mandates strict audit trails for electronic records.
4. Real-Time Data Validation
Integrating MCP with data validation tools allows for real-time detection of inconsistencies or anomalies in data. Immediate identification of issues enables prompt corrective actions, preventing the propagation of errors. This proactive approach to data validation ensures the accuracy and reliability of clinical data throughout the trial process.
Impact of MCP on Regulatory Reporting
Regulatory reporting demands high accuracy and compliance with established standards. MCP contributes to this by:
- Ensuring Compliance: Aligning data interactions with regulatory requirements such as 21 CFR Part 11 and HIPAA.
- Streamlining Reporting Processes: Automating data aggregation and report generation, reducing manual effort and associated errors.
- Facilitating Real-Time Updates: Allowing for timely updates to regulatory bodies, ensuring that the most current data is always available.
Implementing MCP in regulatory reporting processes has led to a 70% reduction in data gathering time and a 50% faster decision-making process in some organizations.
Case Studies and Real-World Applications
Case Study 1: Pharmaceutical Company A
A leading pharmaceutical company integrated MCP into its clinical data management system. As a result, they observed a 50% reduction in data entry errors and a 30% decrease in the time required for data validation processes. This implementation not only improved data quality but also accelerated the overall clinical trial timeline.
Case Study 2: Biotech Firm B
Biotech Firm B adopted MCP to automate regulatory reporting. This implementation led to a 60% decrease in compliance-related discrepancies and improved audit readiness. The streamlined reporting process also enhanced the company’s relationship with regulatory agencies, facilitating smoother approval processes.
Challenges and Considerations
While MCP offers significant benefits, its implementation requires careful planning:
- Integration with Existing Systems: Ensuring that MCP can seamlessly integrate with legacy systems without disrupting operations.
- Training and Adoption: Providing adequate training to staff to effectively utilize MCP tools.
- Continuous Monitoring: Regularly reviewing MCP implementations to identify and address any emerging issues.
Addressing these challenges is crucial for maximizing the potential of MCP in reducing human error in clinical data management and regulatory reporting.
Future Directions
The evolution of MCP continues to shape the landscape of clinical data management and regulatory reporting:
- Advanced AI Integration: Incorporating more sophisticated AI algorithms to enhance data analysis capabilities.
- Expanded Regulatory Compliance: Adapting MCP to meet the requirements of additional regulatory bodies globally.
- Interoperability Enhancements: Improving MCP’s ability to interact with a broader range of data sources and systems.
These advancements promise to further enhance the efficiency and accuracy of clinical data management and regulatory reporting processes.
The Model Context Protocol (MCP) represents a significant advancement in reducing human error within clinical data management and regulatory reporting. By standardizing data interactions, automating processes, and enhancing traceability, MCP contributes to more accurate, efficient, and compliant operations in the pharmaceutical and life sciences industries. As organizations continue to adopt and refine MCP implementations, the potential for further improvements in data integrity and regulatory adherence is substantial.
FAQs
1. How does MCP specifically reduce human error in clinical data management?
MCP automates repetitive tasks, standardizes data inputs, and provides real-time validation. This reduces manual entry errors, improves consistency, and ensures compliance with regulatory standards.
2. Can MCP be integrated with existing clinical trial management systems (CTMS) and EDC platforms?
Yes. MCP is designed to work with APIs and existing data infrastructures. It can integrate with CTMS, EDC, and other regulatory systems without replacing them, making adoption easier for pharma companies.
3. What role does MCP play in regulatory reporting accuracy?
MCP ensures all data is aligned, validated, and traceable before submission. It creates audit trails, supports real-time updates, and automates report generation—leading to fewer compliance discrepancies.
4. What are the main benefits of using MCP in life sciences beyond error reduction?
In addition to minimizing errors, MCP improves efficiency, speeds up trial timelines, ensures audit readiness, and enhances collaboration across teams. It also strengthens trust with regulators by maintaining transparent and traceable processes.
5. What is the future of MCP in pharma and life sciences?
MCP is expected to evolve with AI advancements, providing predictive analytics, better interoperability with global regulatory systems, and stronger support for decentralized clinical trials. Its role will grow as the industry shifts toward automation, digitalization, and error-free compliance.