The Food and Drug Administration (FDA) acts as the vigilant guardian of public health, issuing over 4,000 Form 483s in FY 2024, with the food area leading the way. For businesses in the pharmaceuticals, medical devices, food, and tobacco industries, the FDA’s regulatory reach is ever-present. A significant tool in their enforcement arsenal is the FDA warning letter.
The key is learning how to use the FDA Warning Letters Database effectively. This public resource holds valuable enforcement data that can help businesses shift from reacting to preventing compliance issues before they occur.
TL;DR:
- The FDA Warning Letters database tracks serious compliance violations across industries.
- Act quickly with a clear, step-by-step plan.
- Search by keyword, date, or FDA office to identify common issues like GMP failures or data integrity problems.
- Utilize this information to enhance compliance, prevent violations, and develop effective responses to warning letters.
However, before diving into the database, let’s grasp the essence of an FDA warning letter.
What Do You Mean by FDA Warning Letter?
An FDA warning letter is a formal notice that signals serious regulatory violations, not just recommendations. It is issued to a company that has violated U.S. regulations in a significant way or if its response to the FDA’s Form 483 is inadequate. An FDA Form 483 lists specific observations made by inspectors during a facility inspection, highlighting potential compliance issues requiring corrective action.
Usually, you have 15 business days to respond and explain your corrective actions. Failure to respond promptly may result in escalated enforcement like import restrictions, legal injunctions, product confiscation, or criminal prosecution.
Note: A Form 483 is issued after an inspection to highlight potential violations observed, whereas a Warning Letter is a formal notification issued after the FDA reviews the company’s response to the Form 483, signaling more serious compliance issues requiring corrective action.
Types of Warning Letters
There are usually three types of warning letters, which are as follows:-
- General FDA Warning Letters: These are official notices sent by the FDA when significant violations of federal laws related to product safety, labeling, manufacturing, or marketing are identified.
- Tobacco Retail Warning Letters: Issued after compliance inspections under the Tobacco Control Act, mainly targeting retailers violating rules on the sale and distribution of cigarettes and smokeless tobacco.
- Drug Marketing and Advertising Warning Letters: Issued for misleading marketing claims by pharmaceutical companies. This includes “cyber letters” sent to online pharmacies selling unapproved or illegal prescription drugs.
Notes: You may encounter an advisory letter, which is a less formal communication that guides without enforcement implications, whereas a warning letter is a formal notice indicating serious regulatory violations that require prompt corrective action.
Common Violations You Might See:
- Good manufacturing practice (GMP) Failures: Issues like poor quality control, missing records, or faulty equipment. In FY2023, over half of the medical device warning letters cited issues such as inadequate CAPA procedures or incomplete medical device reporting processes.
- Mislabeling or False Claims: This happens when products make unapproved or unsupported claims.
- Data Integrity Issues, such as missing, falsified, or inaccurate records, have become a growing concern for the FDA since 2014 due to their critical impact on product safety and regulatory trust.
- Unapproved Products: Selling products without the required FDA approval or clearance.
In FY2024 alone, the FDA issued 738 warning letters to drug and biologicsmanufacturers, with a particular focus on over-the-counter (OTC) products and hand sanitizers, illustrating how the FDA’s priorities shift in response to industry risks.
Understanding how warning letters work and interpreting FDA expectations enables you to strengthen compliance and avoid similar risks. The FDA Warning Letters Database helps you track violations, spot patterns, and stay within regulations.
Also Read: Common FDA Violations and Warning Letters Explained
Why the FDA Warning Letters Database Matters
The FDA’s warning letters database is a public tool designed to promote transparency and drive voluntary compliance. For businesses, it’s a valuable source of insight. For example, if you’re launching a new medical device, searching the database shows warning letters sent to similar companies. You’ll quickly identify common issues such as poor design validation, inadequate complaint handling, or quality system gaps, helping you avoid the same mistakes.
Master the FDA Warning Letters Database: Step-by-Step Guide
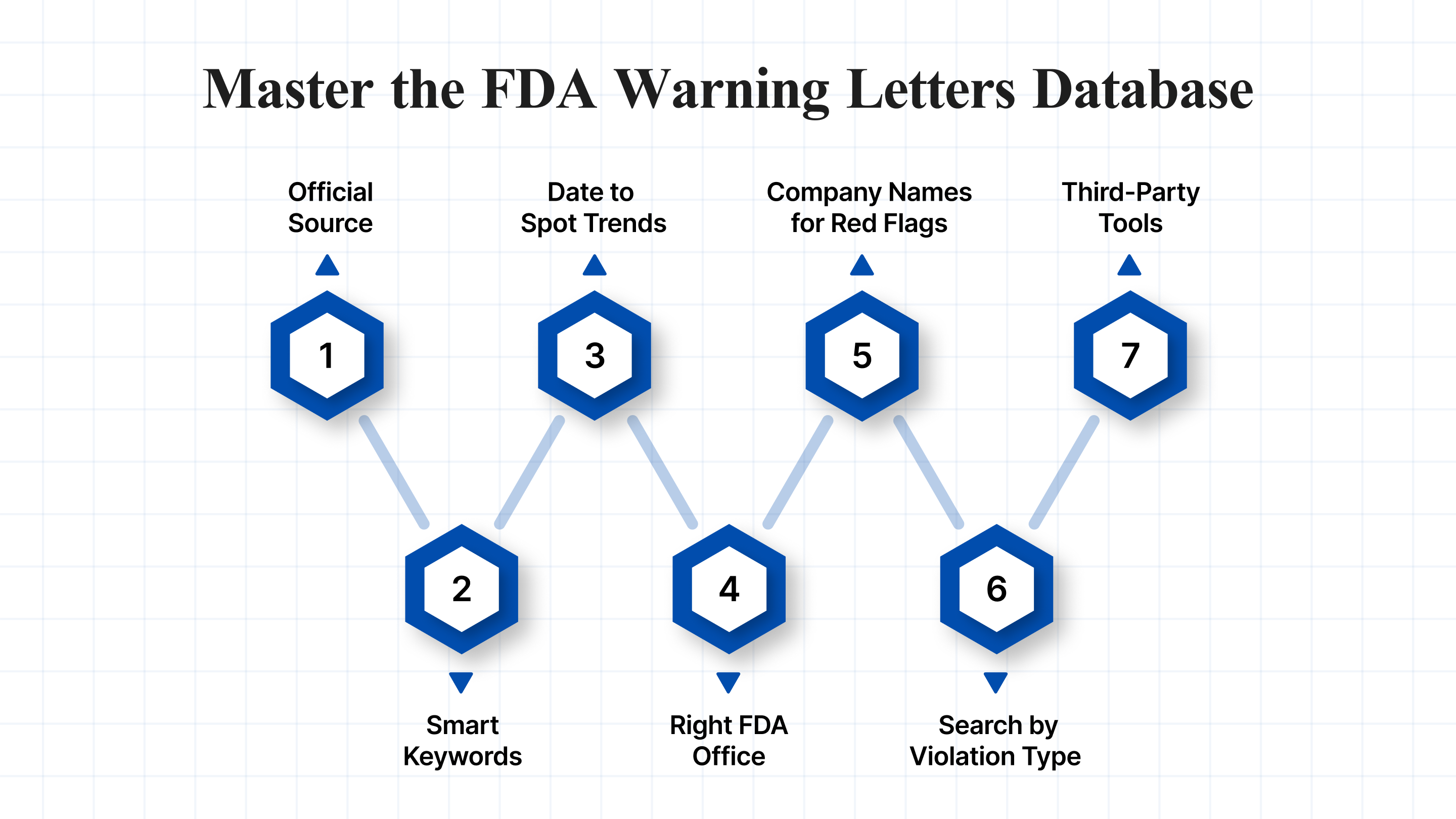
Learn how to use the FDA Warning Letters Database efficiently to spot risks and maintain compliance.
1. Start at the Official Source
Go directly to the FDA Warning Letters page. Avoid relying on third-party summaries at this stage; instead, obtain the most accurate and up-to-date data directly from the FDA website.
2. Use Smart Keywords
Search with terms that match your industry or compliance concerns. Examples:
- “GMP” for manufacturing quality issues.
- “Medical device” for device-related warnings.
- “Dietary supplement” for labeling or safety violations.
- “Data integrity” for documentation failures.
3. Filter by Date to Spot Trends
Review recent letters (from the past 1–2 years) to see where the FDA is currently focusing. For instance, 53% of drug-related inspection warning letters in FY2024 pertained to OTC products, a clear indication of shifting priorities.
4. Focus on the Right FDA Office
Refine your search by the issuing office:
- CDRH: Medical Devices
- CDER: Drugs
- CFSAN: Food and Supplements
- ORA: Office of Regulatory Affairs (broad inspections).
It helps you understand what’s relevant to your product category.
5. Check Company Names for Red Flags
Look up competitors, suppliers, or potential partners; multiple warnings indicate potential compliance risks that could affect business relationships, so proceed with caution or conduct further investigation.
6. Search by Violation Type (CFR Citations)
Use the Code of Federal Regulations (CFR), the set of federal rules cited in warning letters, to pinpoint specific violations and tailor your corrective actions to meet FDA legal requirements relevant to your industry.
7. Go Beyond the Basics with Third-Party Tools
Platforms like Atlas Compliance analyze historical FDA data and recall information to identify emerging compliance risks, enabling businesses to implement preventive measures proactively.
These tools provide a broader, more comprehensive view of compliance risks across the industry, rather than focusing on one letter at a time.
Pro Tip: Regularly monitoring this database isn’t just about avoiding penalties; it’s a roadmap for proactive compliance. Staying ahead of common violations can save your business from costly mistakes.
How to Interpret the Warnings on the Letter?
A warning letter isn’t just a checklist of problems; it reflects how the FDA interprets the rules and expects you to fix issues. Focus on:
- Citations: These point to the exact regulations you violated.
- Observations: The FDA explains what they found and why it matters, which is essential for finding the root cause.
- Action Requests: Many letters suggest clear steps, such as hiring a cGMP consultant, which is noted in 60 of 94 drug-related letters in FY2023.
- Strict Deadlines: You must respond within 15 business days, or the situation can escalate quickly.
For example: Warning Letter to Zhejiang Huahai Pharmaceutical Co., Ltd. Date Issued: June 06, 2025, FEI: 3003999190
This letter, issued by the Center for Drug Evaluation and Research (CDER), states “Your firm failed to clean, maintain, and, as appropriate for the nature of the drug, sanitize and/or sterilize equipment and utensils at appropriate intervals to prevent malfunctions or contamination that would alter the safety, identity, strength, quality, or purity of the drug product beyond the official or other established requirements (21 CFR 211.67(a)).”
In the letter, the Regulatory Citation (21 CFR 211.67(a)) indicatesthat the company has violated the regulation concerning equipment cleaning and maintenance. It is a foundational cGMP requirement to prevent contamination and ensure product quality.
Let’s understand that the next step is using that knowledge to strengthen your compliance and avoid similar risks.
Also Read: FDA Issues and KVK-Tech Warning Letters Explained
How to Apply What You Find
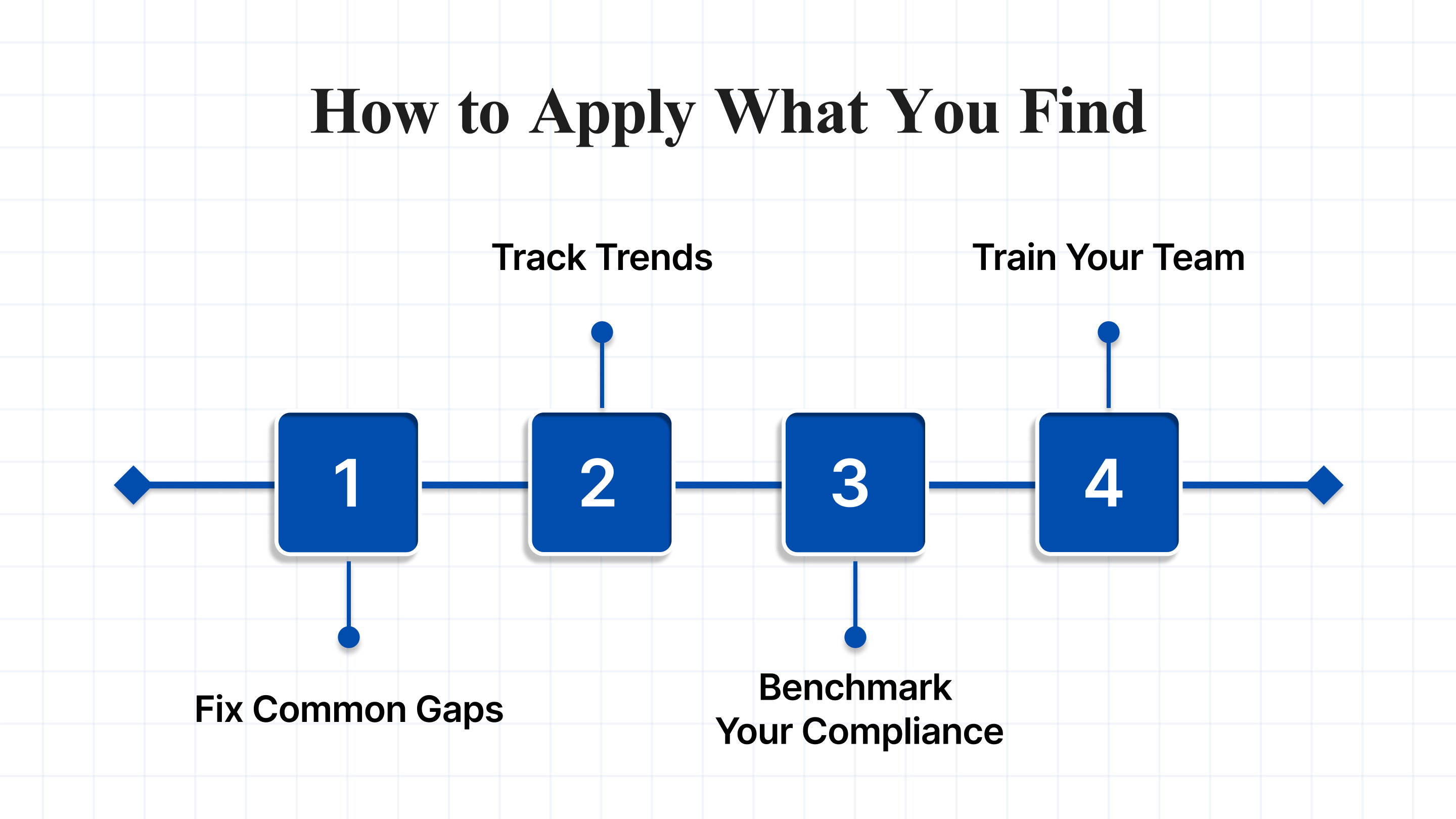
Using the FDA Warning Letters Database isn’t just about looking up violations. Apply what you learn to:
- Fix Common Gaps: If multiple companies get flagged for poor CAPA processes, review yours now.
- Track Trends: Recent letters show a rising focus on issues like diethylene glycol in OTC drugs; stay alert.
- Benchmark Your Compliance: Compare your practices with those of others in your industry to identify areas for improvement.
- Train Your Team: Real cases make excellent training tools to avoid mistakes others have made.
If you ever receive an FDA warning letter, responding swiftly and appropriately is critical to protecting your business and avoiding further action.
How to Respond to an FDA Warning Letter
Key steps to respond to an FDA warning letter include:
- Acknowledge the Letter: Inform the FDA that you’ve received the notice and that a response is forthcoming.
- Investigate the Root Cause: Find out exactly why the violations happened. This step is crucial for lasting fixes.
- Create a CAPA Plan: Outline detailed corrective and preventive actions for each violation. Include deadlines and who is responsible.
- Submit Proof: Share documents that show what changes have been made and how you’re monitoring progress.
- Keep the FDA Updated: Stay in regular contact and report progress on your CAPA plan.
The end goal is to earn a close-out letter, confirming the FDA accepts your corrections and the matter is resolved. That’s where Atlas Compliance acts as your proactive partner, helping you identify, fix, and prevent regulatory issues before they escalate.
Why Choose Atlas Compliance for FDA Readiness?

Atlas Compliance helps pharmaceutical, medical device, and life sciences companies stay one step ahead of FDA inspections. It simplifies how teams track, manage, and respond to compliance risks, making inspection readiness more efficient and proactive.
Key Advantages:
- Real-Time FDA Insights: Instantly access updated data on inspections, 483s, warning letters, and CFR references.
- AI-Driven Search: Find precise regulatory information fast with natural language processing, no tedious searching.
- Predictive Compliance Monitoring: Machine learning algorithms analyze compliance data patterns to identify early warning signs of potential risks, enabling proactive responses.
- Wide Industry Support: Serves pharma, biologics, medical devices, cosmetics, food, and more.
- Supplier Risk Management: Monitor and assess third-party compliance in a single, centralized location.
- All-in-One Platform: Connects Quality, Compliance, and Regulatory teams for effortless oversight.
Atlas Compliance gives your team the confidence to stay audit-ready, minimize regulatory risks, and avoid costly penalties, all without guesswork.
Final Thoughts
The FDA Warning Letters Database isn’t just an enforcement record—it’s a powerful tool for learning and strengthening compliance. Knowing how to search, analyze, and apply insights from past violations helps businesses lower regulatory risks and maintain safe, compliant operations.
As the FDA shifts toward stricter, data-driven enforcement, staying compliant is no longer optional; it’s essential. Whether preparing for audits, evaluating suppliers, or launching products, using warning letter insights is a smart move.
Atlas Compliance helps you stay proactive, reduce risks, and meet FDA expectations with confidence. Book a demo today and take control of your compliance journey.
FAQs
Q1. What is an FDA Warning Letter?
A formal notice from the FDA, signaling serious regulatory violations and demanding prompt corrective action.
Q2. Why is the FDA Warning Letters database useful?
It offers transparency into FDA enforcement, allowing businesses to learn from past violations and proactively improve compliance.
Q3. How long do I have to respond to an FDA Warning Letter?
Typically, you have 15 business days to submit a comprehensive response detailing your corrective and preventive actions.
