Staying compliant with FDA regulations can be challenging, especially when inspection outcomes are complex and unclear. For regulated companies, understanding FDA inspection classifications is key to managing risk and staying audit-ready.
We recognize that interpreting FDA inspection results and keeping up with shifting regulations can feel overwhelming for MedTech, pharmaceutical, and life sciences companies. Staying compliant under constant regulatory pressure is no small task.
The FDA inspects facilities to assess compliance with laws such as the Food, Drug, and Cosmetic Act (FD&C Act). These inspections result in one of three classifications: No Action Indicated (NAI), Voluntary Action Indicated (VAI), or Official Action Indicated (OAI). Each classification reflects the firm’s level of regulatory compliance.
These classifications signal compliance status and may prompt enforcement actions. The FDA publishes selected inspection data weekly through itsInspections Data Dashboard to promote transparency.
Some details may be withheld until final actions are taken, and posted citations may differ from final classification letters. In this blog, we’ll explain what each classification means and why it matters for compliance and quality assurance.
TL;DR
- FDA inspections result in NAI, VAI, or OAI classifications, reflecting a facility’s compliance status.
- NAI indicates full compliance, VAI signals minor issues, and OAI means serious violations needing FDA action.
- Common triggers for VAI/OAI include outdated SOPs, data integrity issues, sanitation lapses, and labeling errors.
- Proactive compliance measures like strong CAPA systems and updated procedures help prevent adverse classifications
What is the FDA Inspection Database?
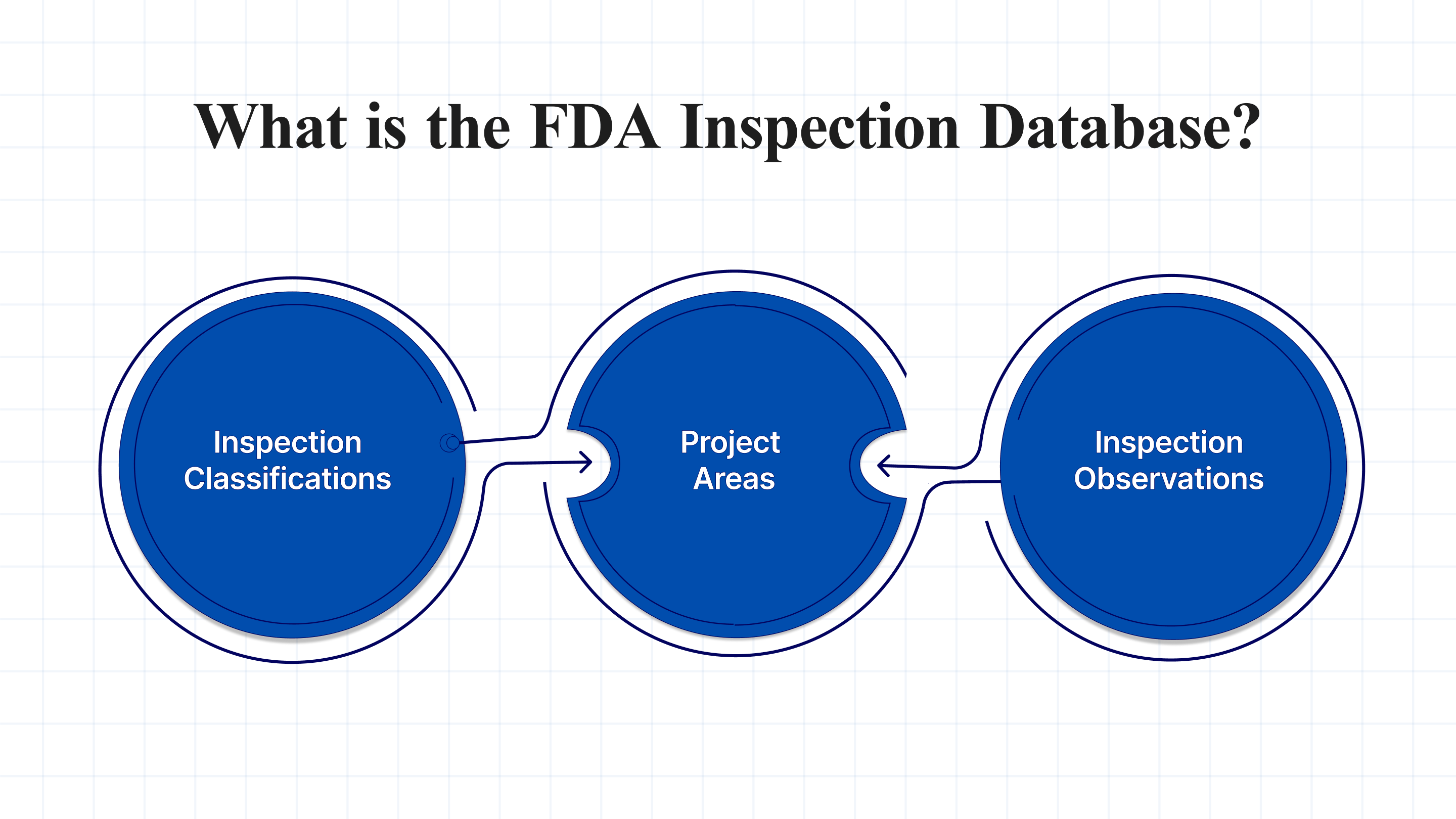
The FDA Inspection Database, officially known as the Inspection Classification Database, is a publicly accessible resource maintained by the U.S. Food and Drug Administration (FDA). It provides information on the outcomes of FDA inspections conducted at facilities regulated by the agency. These inspections assess compliance with laws such as the Federal Food, Drug, and Cosmetic Act.
Here are the core elements that make the database valuable for tracking regulatory status and compliance history:
- Inspection Classifications: Each inspection is assigned a classification based on findings, such as NAI, VAI, and OAI.
- Project Areas: Inspections may cover multiple areas, such as drug quality assurance or over-the-counter drug evaluation. Each area is classified separately.
- Inspection Observations: If investigators observe conditions that may violate FDA regulations, they document these on Form FDA 483. These observations are part of the inspection record and may lead to further actions.
Together, these components offer critical insight into a facility’s regulatory standing and help stakeholders monitor trends in FDA compliance across the industry.
Why is the FDA Inspection Database Important?
The FDA Inspection Database is a crucial resource that contains detailed information about FDA inspections, providing insight into how well companies follow FDA regulations and the quality of products they produce. Below are several reasons why the FDA Inspection Database is important.
- Promotes Transparency: Makes inspection data publicly available, ensuring accountability and building trust with consumers, businesses, and regulators.
- Informs Decision-Making: Helps healthcare providers, businesses, and consumers choose safe and compliant products based on inspection outcomes.
- Supports Regulatory Actions: Provides data for issuing warning letters, product recalls, and import alerts, guiding enforcement decisions.
- Aids Research & Policy Development: Helps researchers and policymakers analyze compliance trends, identify risks, and shape future regulations.
- Improves Risk Management: Allows businesses to assess the regulatory status of suppliers, ensuring safe and compliant partnerships.
- Monitors Compliance Over Time: Tracks ongoing adherence to safety standards, ensuring corrective actions are effective and preventing repeated violations.
- Improves Industry Standards: Provides a benchmark for best practices and encourages facilities to maintain high compliance levels.
Moreover, the inspected firms are urged to submit a written response within 15 days of receiving a Form FDA 483, FDA 4056, or verbal observations. This response should include a corrective action plan with supporting documentation, and corrective actions should be implemented promptly to address the cited issues.
Understanding the Three FDA Inspection Classifications
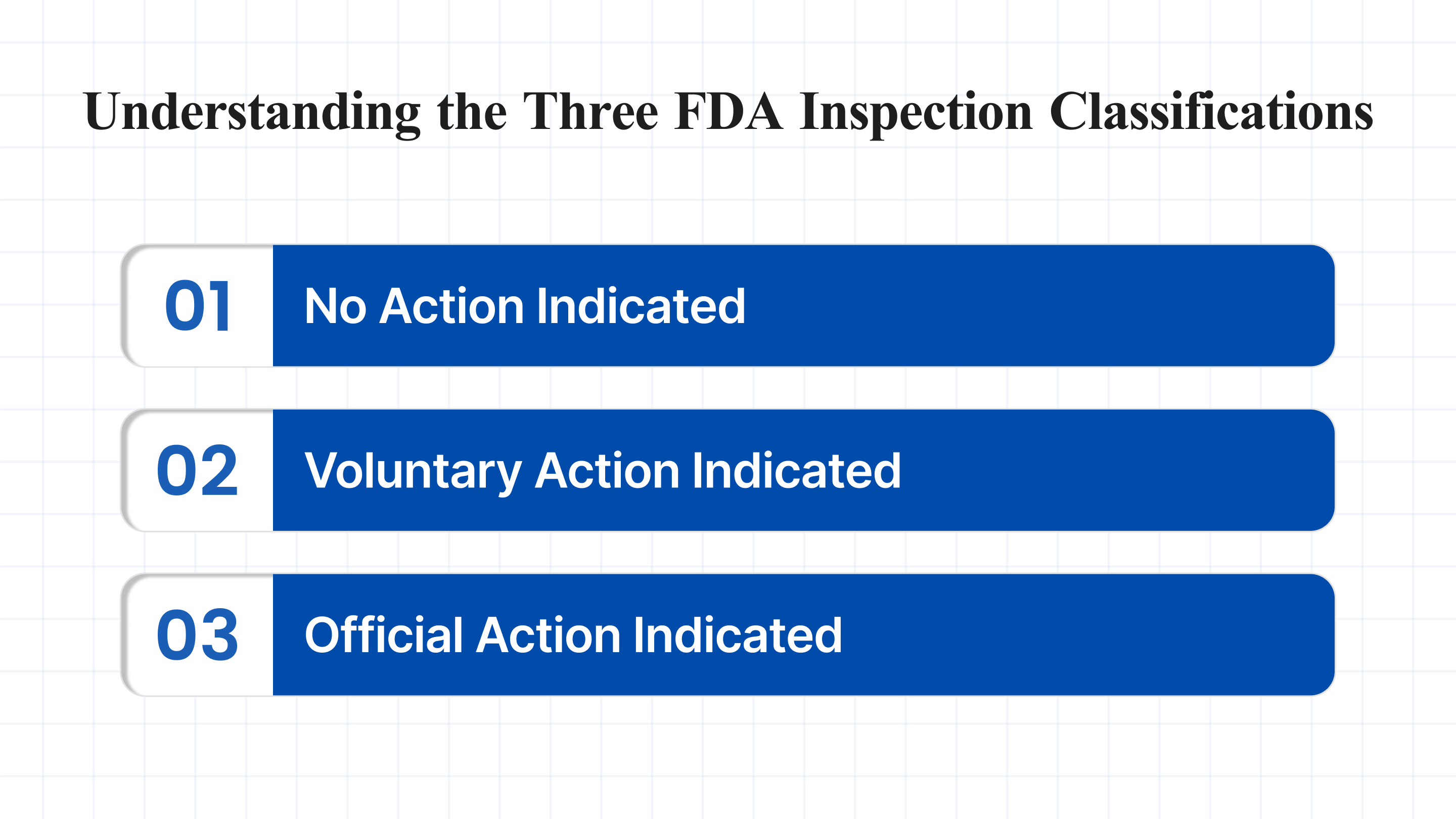
Understanding the three main FDA inspection classifications —NAI, VAI, and OAI —is essential for interpreting inspection outcomes and ensuring regulatory readiness. Below is a concise summary, followed by detailed explanations and examples of each classification.
| Classification | Meaning | Severity | Action Required | Example |
|---|---|---|---|---|
| NAI | No Action Indicated | Low | None | Fully GMP-compliant pharma site |
| VAI | Voluntary Action Indicated | Moderate | Voluntary correction | Minor labeling or documentation gaps |
| OAI | Official Action Indicated | High | Formal FDA action is likely | Contamination or falsification of records |
1. NAI (No Action Indicated)
This is the best outcome a company can receive. It means that the FDA inspector found no significant issues during the inspection. No further regulatory action is required, and the company is considered to be in full compliance with FDA regulations. However, it’s important to remember that NAI doesn’t mean the company is free from future inspections or obligations. It’s simply a sign that, at the time of inspection, everything was in order.
Example: A pharmaceutical company undergoing an FDA inspection may receive an NAI classification if the inspector finds that all manufacturing processes are fully compliant with GMP and no violations are noted.
2. VAI (Voluntary Action Indicated)
This rating indicates that the FDA found some minor issues that don’t immediately threaten public health but require attention. While no formal enforcement action is necessary, the company is expected to take voluntary corrective measures to address the issues noted in the inspection report. VAI is essentially a warning, and companies need to respond promptly to avoid escalating issues.
Typically, the facility received a Form FDA 483 or FDA 4056 at the conclusion of the inspection.
Example: A food manufacturer might receive a VAI if they have minor labeling discrepancies that could potentially mislead consumers but don’t pose a direct health risk.
3. OAI (Official Action Indicated)
This is the most serious classification. It means that the inspector identified significant violations that require immediate corrective action, such as fines, penalties, or even product recalls. OAI often leads to official FDA actions like warning letters, import alerts, or enforcement actions. A company receiving this classification must act quickly to address the violations to avoid further legal consequences. The facility may have received a Form FDA 483 or FDA 4056 at the end of the inspection.
Example: A vaccine manufacturer might receive an OAI classification if the inspection uncovers a serious deviation from GMP, such as contamination in a batch of vaccines, which could compromise public health.
The FDA typically issues its final inspection classification in an official letter to the firm within 45 to 90 days after the inspection concludes; this period allows time for review of inspection findings, firm responses, and regulatory considerations based on inspection complexity.
Why Do These Classifications Matter?
Understanding your FDA inspection classification is more than just a regulatory checkbox; it has a direct impact on your company. Here’s how:
- Regulatory Impact: An OAI classification can trigger warning letters, import alerts, or license suspensions.
- Business Risk: VAI or OAI findings can delay product launches, damage brand trust, or interrupt supply chains.
- Reputation & Partnerships: Clients, partners, and investors often check FDA inspection history when evaluating a company.
- Continuous Compliance Strategy: Responding appropriately to inspection results is critical to maintaining long-term regulatory health.
Atlas Compliance is a regulatory intelligence platform that helps companies manage and monitor FDA inspection data. We ensure that companies can streamline preparation, stay ahead of inspection trends, and respond effectively to FDA findings.
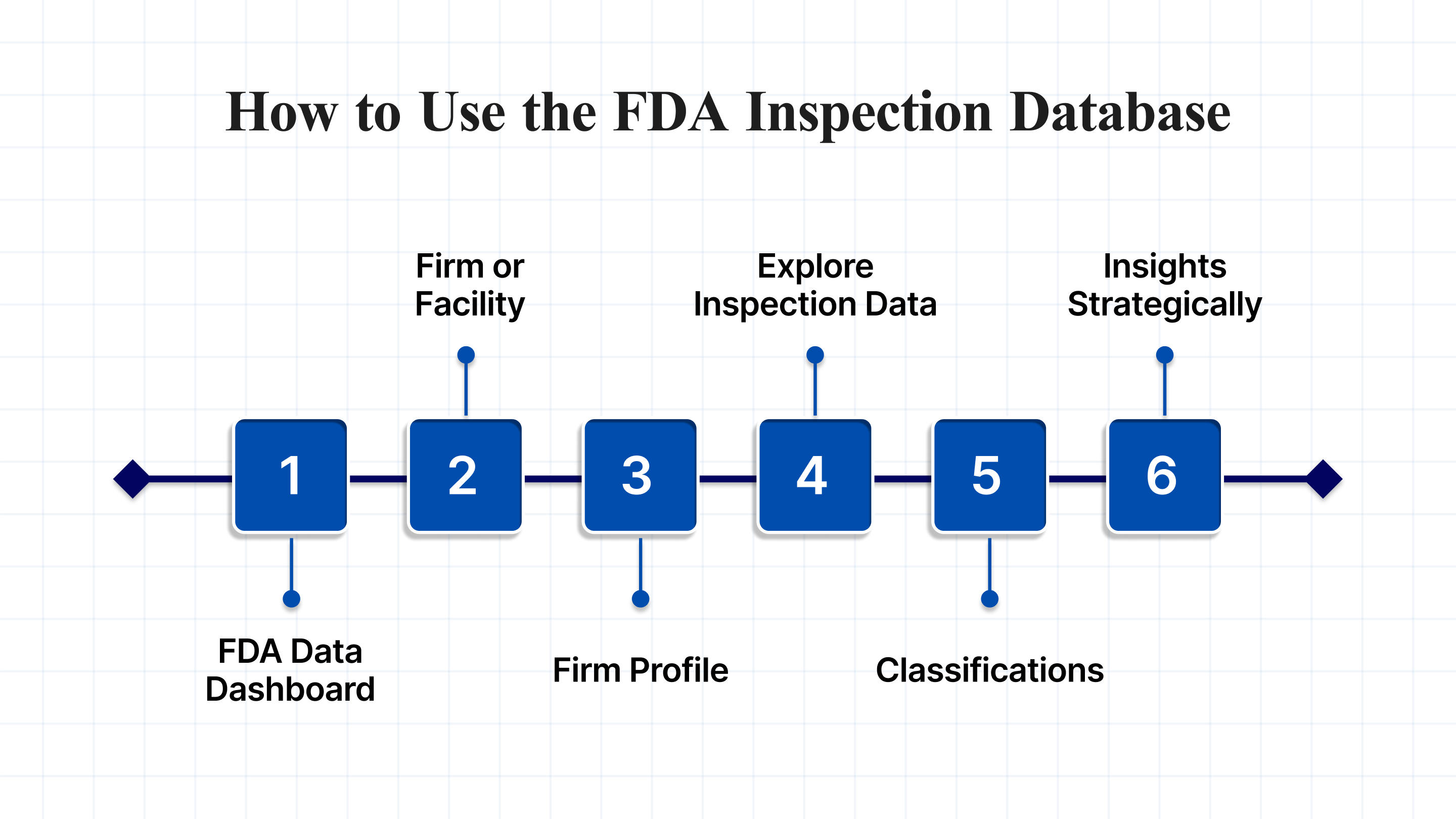
How to Use the FDA Inspection Database
The FDA Inspection Classification Database is a critical tool for anyone involved in regulatory compliance, quality assurance, or supply chain risk management. It offers transparency into inspection outcomes at facilities regulated by the U.S. Food and Drug Administration (FDA), including drug, device, food, and biologics manufacturers.
Whether you’re assessing a supplier, preparing for your own inspection, or tracking industry trends, here’s how to get the most out of the database.
1. Access the FDA Data Dashboard
The FDA provides a public and user-friendly online platform called the FDA Data Dashboard, which compiles inspection data, including inspection classifications, Form 483 citations, warning letters, and import alerts. This online tool is free and publicly available. No registration or login is required.
2. Search for a Firm or Facility
Use the search bar to enter the firm name or Facility Establishment Identifier (FEI). The dashboard will return matching firms, allowing you to select the specific entity of interest.
3. Review the Firm Profile
Once a firm is selected, you can view detailed information such as:
- Latest firm details (name, address, FEI)
- Timeline of compliance actions (inspections, recalls, import refusals)
- Inspection outcomes and final classifications (No Action Indicated, Voluntary Action Indicated, Official Action Indicated)
- Links to related documents like Warning Letters and Import Alerts
Clicking on an entry may provide more detailed insights or allow you to cross-reference with other FDA databases, such as Warning Letters or 483 Observations.
4. Filter and Explore Inspection Data
The dashboard offers interactive charts, maps, and tables that update dynamically based on your filters. You can filter by inspection date, location, inspection type, or outcome to analyze trends or specific data points.
5. Understand the Classifications
Each classification tells you how the FDA viewed the inspection findings:
- No Action Indicated (NAI): No objectionable conditions were found. The facility is considered in compliance.
- Voluntary Action Indicated (VAI): Some issues were observed, but not severe enough to trigger enforcement. The FDA expects the company to take corrective actions voluntarily.
- Official Action Indicated (OAI): Serious violations were identified. This classification may lead to warning letters, import alerts, injunctions, or other formal enforcement actions.
6. Apply Insights Strategically
The FDA Inspection Database isn’t just for checking your records; it can be a powerful tool to support broader regulatory and business decisions:
- Supplier Evaluation: Check the compliance history of contract manufacturers, raw material providers, or testing labs.
- Inspection Readiness: Study recent inspections within your industry to understand common findings and prepare accordingly.
- Risk Assessment & Due Diligence: Use inspection data during mergers, acquisitions, or vendor onboarding to assess regulatory risk.
- Benchmarking: Compare your inspection outcomes against others in the industry to identify improvement areas.
- Regulatory Monitoring: Stay current on FDA trends in enforcement and policy focus across regions or therapeutic areas.
By understanding how to use this data strategically, you can proactively manage compliance risks and avoid the pitfalls that often lead to unfavorable inspection outcomes.
Common Reasons for VAI and OAI Classifications
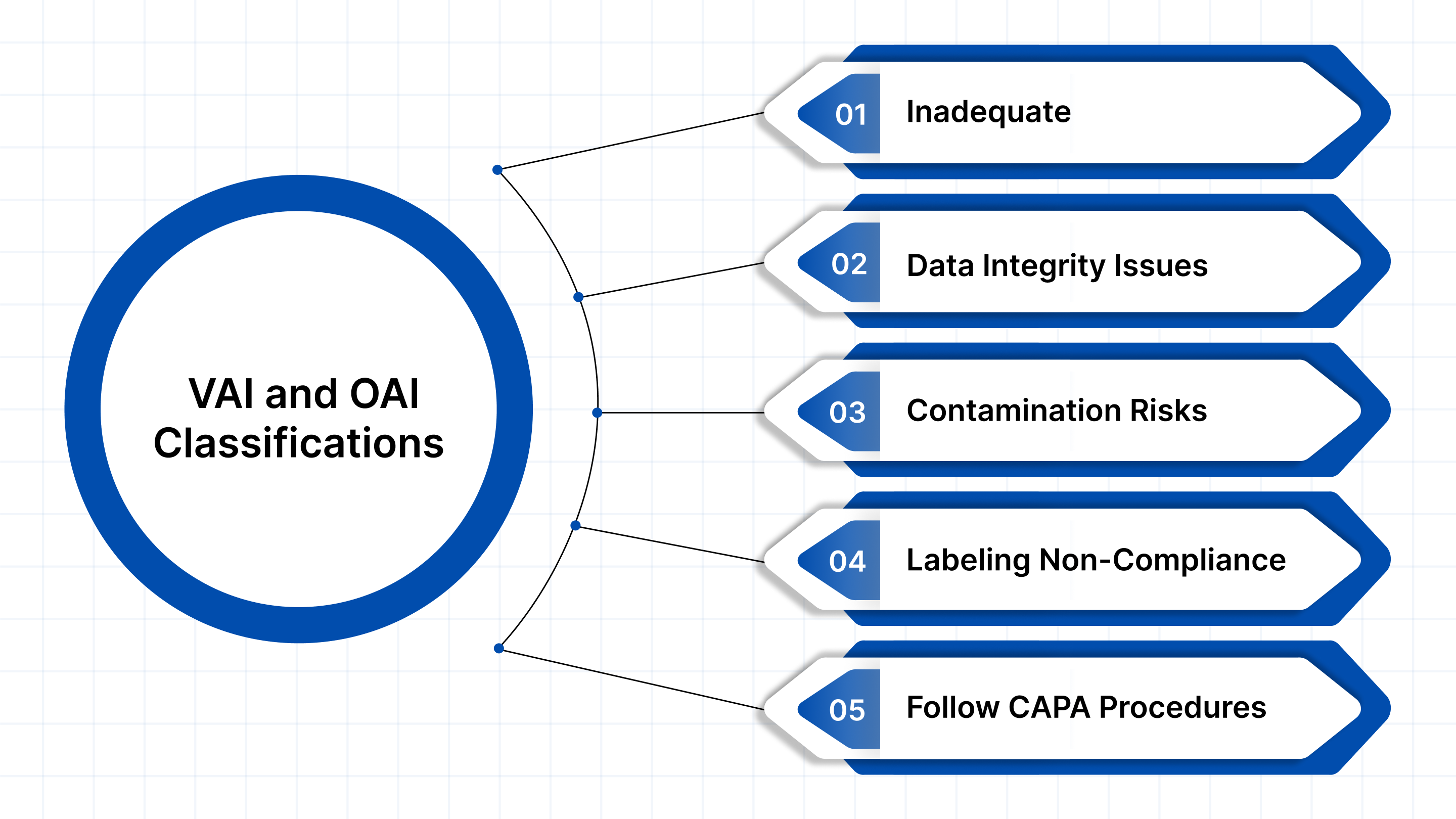
Avoiding VAI and OAI outcomes begins with understanding the root causes that typically lead to them. The FDA’s focus during inspections is on whether your facility meets current Good Manufacturing Practices (cGMPs), ensures product safety, and maintains data integrity. Below are some of the most common triggers for adverse inspection outcomes:
1. Inadequate or Outdated Standard Operating Procedures (SOPs)
SOPs are the foundation of compliant operations. If SOPs are outdated, inconsistent with current practices, or missing altogether, inspectors will issue Form 483 observations. Poorly written or rarely reviewed SOPs reflect weak quality systems and operational oversight.
2. Data Integrity Issues
The FDA places high importance on the reliability of data. Common violations include:
- Altered or backdated records
- Missing audit trails in electronic systems
- Lack of restricted access or user accountability
These red flags suggest intentional misconduct or inadequate controls and often lead directly to an OAI.
3. Sanitation or Contamination Risks
Unclean manufacturing environments or poor sterile practices pose a direct threat to product safety. In the case of biologics, injectables, or food, contamination risks are taken very seriously and may result in immediate enforcement actions.
4. Labeling Non-Compliance
Mislabeling is a frequent cause of VAI ratings. Errors in dosage instructions, incorrect ingredient listings, or unapproved marketing claims can lead to consumer harm. These violations often start small but may escalate to OAI if found systemic or repeated.
5. Failure to Follow CAPA Procedures
CAPA systems are vital for long-term compliance. Failing to investigate previous issues, delaying corrective actions, or not verifying effectiveness can signal a breakdown in your quality management system. If a facility ignores past findings, the FDA views it as a serious compliance risk.
Note: Facilities that receive repeat observations for similar issues are at higher risk of moving from VAI to OAI status in subsequent inspections.
Recognizing these common pitfalls is only the first step, and then proactively addressing them through strong quality systems and ongoing improvement is key to maintaining a strong compliance record.
Best Practices to Avoid VAI and OAI Outcomes
Reducing the risk of adverse FDA classifications requires more than reactive fixes, it takes proactive, systematic compliance management. Below are proven strategies to help maintain a state of inspection readiness and reduce the likelihood of receiving a VAI or OAI outcome.
1. Keep SOPs Current and Effective
Outdated or poorly written Standard Operating Procedures (SOPs) are a frequent root cause of Form 483 observations. To avoid this, regularly review and revise SOPs to ensure they reflect actual current practices, regulatory updates, and operational changes.
Provide ongoing training to all relevant staff and verify that procedures are consistently followed through internal audits or real-time oversight. Well-maintained SOPs demonstrate operational control and commitment to quality.
2. Ensure Strong Data Integrity Controls
To avoid data-related enforcement actions, implement strong electronic and manual systems that support full traceability and accountability. This includes using software that maintains complete audit trails, limits system access by user roles, and prevents unauthorized changes. Train staff on proper documentation practices and electronic record handling to ensure data is contemporaneous, accurate, and attributable, a key pillars of FDA’s expectations.
3. Implement Strict Sanitation and Environmental Controls
Especially in sterile manufacturing, biologics, and food environments, sanitation failures can quickly escalate to serious compliance concerns. Establish and document rigorous cleaning protocols, ensure personnel follow aseptic techniques, and perform routine environmental monitoring. Proactive measures like internal hygiene audits can help catch potential issues early, reducing the likelihood of microbial contamination findings that trigger OAI outcomes.
4. Conduct Ongoing Labeling Reviews
Labeling violations, such as incorrect dosage instructions or unauthorized claims, often result in VAI findings. To prevent this, regularly audit product labels to ensure alignment with approved specifications, regulatory submissions, and marketing content. Use a formal change control system to manage label updates and prevent unapproved modifications from reaching the market.
5. Strengthen CAPA Processes
A weak Corrective and Preventive Action (CAPA) system often signals systemic quality failures to inspectors. Ensure each CAPA is supported by a thorough root cause investigation, and implement meaningful corrective actions, not just temporary fixes. Monitor the effectiveness of each action taken, and document all steps to provide clear evidence of resolution and preventive controls. A mature CAPA system demonstrates a strong, self-correcting quality culture.
6. Utilize Compliance Technology
Investing in compliance tools can significantly reduce manual oversight burdens and improve inspection preparedness.
Platforms like Atlas Compliance help companies:
- Monitor FDA inspection trends across facilities and competitors
- Track internal observations and identify repeat issues
- Use AI-powered tools to flag early signs of noncompliance
- Centralize quality documentation for quick, audit-ready access
- Stay informed about FDA enforcement updates and regulatory changes
Using digital tools proactively supports continuous improvement and reduces the likelihood of findings going unnoticed until the next inspection.
How Atlas-Compliance Supports Success in FDA Inspection Outcomes?
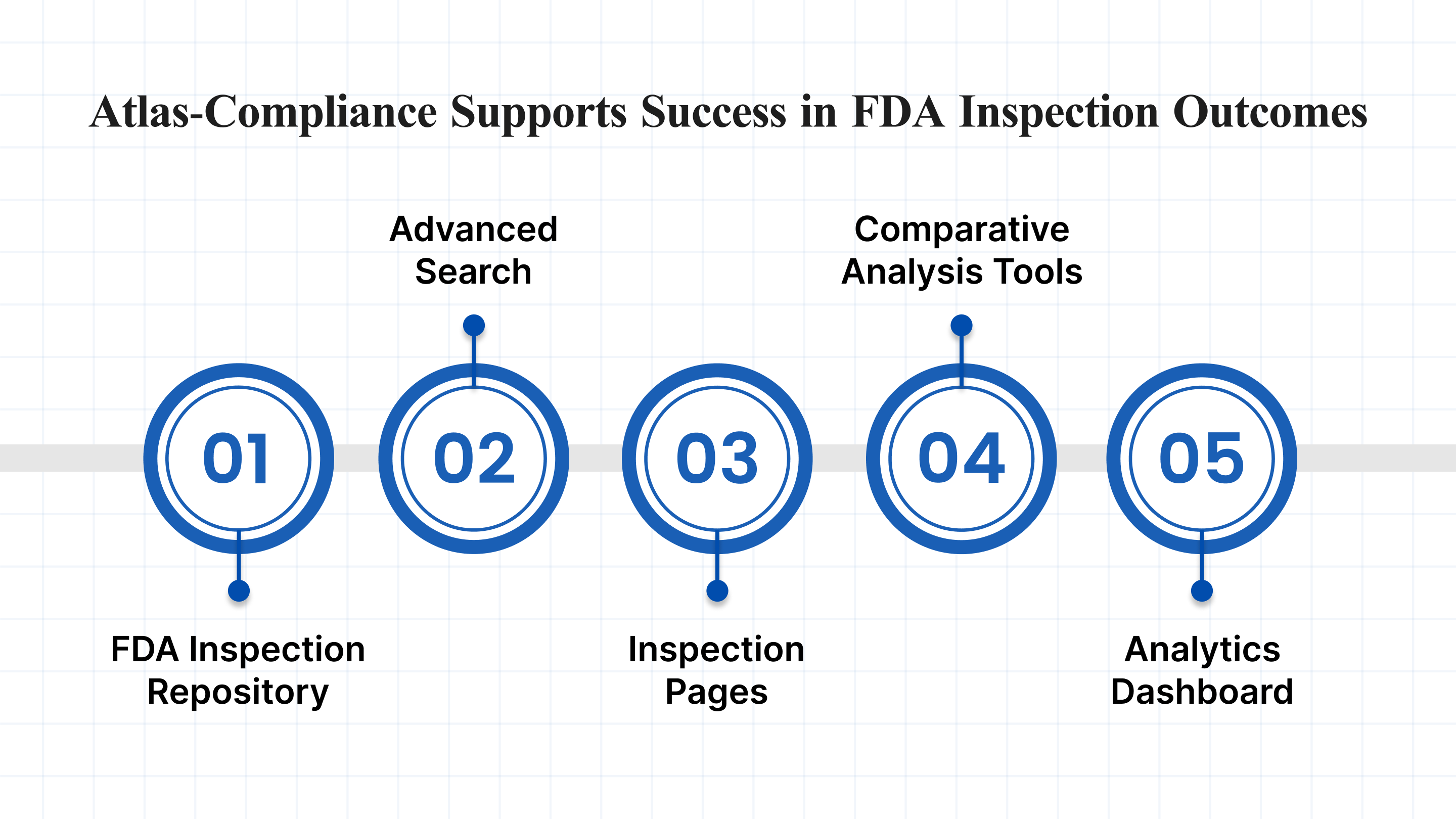
While the FDA Inspection Database provides historical inspection data, Atlas Compliance serves as an advanced compliance intelligence database that transforms static information into real-time, actionable insights.
By layering analytics, machine learning, and regulatory monitoring on top of inspection data, Atlas Compliance helps organizations move from passive tracking to strategic, proactive compliance management.
Key features of Atlas Compliance include:
1. Extensive FDA Inspection Repository: Access a strong archive of FDA inspection records dating back to 2010. The database is refreshed every 20 business days to include newly released inspections. Gain early access to Form FDA 483s, including some documents available before they appear in public databases.
2. Advanced Search and Filtering Options: Quickly find relevant inspection records using powerful filters, including:
- Site Name
- Facility Establishment Identifier (FEI ID)
- Country and Region
- Product Category (e.g., Human Drugs, Tobacco, Food & Cosmetics)
- Project Area or GxP Focus (GMP, GCP, GLP, etc.)
- Form 483 Issuance and Warning Letter Status
3. In-Depth Inspection Pages: Each inspection profile includes:
- Inspection dates, durations, and classification outcomes
- Associated CFR citations and regulatory focus areas
- Status of Form 483s or Warning Letters
- Download links for available documents, or the ability to request unavailable ones
4. Comparative Analysis Tools: Evaluate internal facilities, suppliers, or competitors using built-in comparison features:
- Ratios of inspections to issued Form 483s
- Product recall frequency and root causes
- Classification trends across time and categories (NAI, VAI, OAI)
5. Interactive Analytics Dashboard: Make data-driven decisions with real-time visual insights, including:
- Inspection volumes by geography, product type, or project area
- Trends in FDA enforcement outcomes
- Recalls, Form 483 citations, and Warning Letter summaries
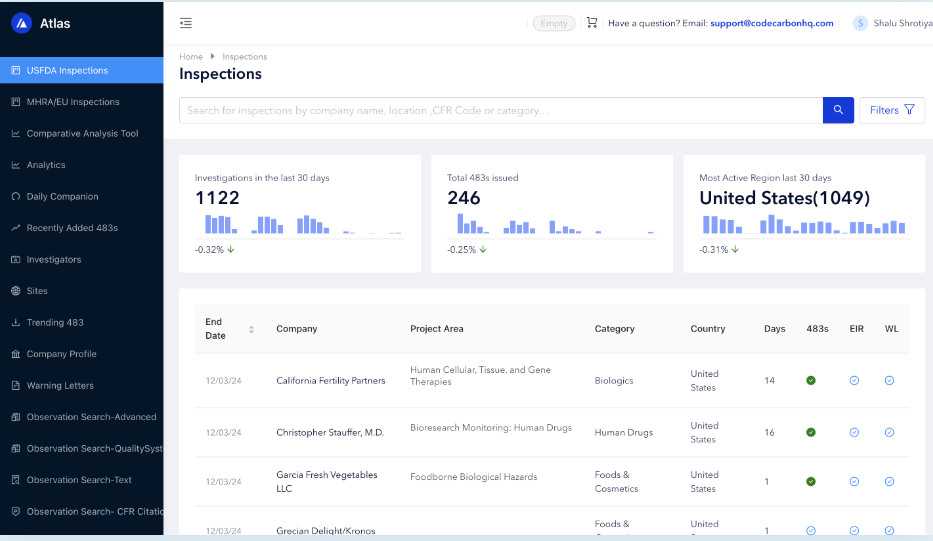
With Atlas Compliance, you get more than just inspection records. You gain a powerful regulatory intelligence platform that helps you prepare, benchmark, and protect your business.
Conclusion
Understanding the outcomes of FDA inspection databases, such as NAI, VAI, and OAI, is essential for companies in regulated industries. To avoid negative classifications, organizations must prioritize continuous compliance, stay updated on regulatory changes, and maintain strong audit readiness. However, achieving this level of preparedness can be challenging without the right tools and systems in place.
This is where Atlas Compliance provides a practical solution, streamlining compliance workflows, centralizing documentation, and helping teams stay inspection-ready at all times.
Ready to strengthen your compliance efforts and minimize inspection risks?
Book your free demo today to learn how our platform can streamline your regulatory processes and keep your organization audit-ready at all times.
FAQs
Q1. What is the significance of receiving an NAI classification from the FDA?
A1.NAI (No Action Indicated) means the FDA found no significant violations during the inspection. It’s the best outcome and indicates full compliance.
Q2. Can I still face an FDA inspection after receiving an NAI rating?
A2. Yes, receiving an NAI rating does not mean you are exempt from future inspections. It simply reflects compliance at the time of inspection.
Q3. What should I do if I receive a VAI or OAI classification?
A3. If you receive a VAI (Voluntary Action Indicated) or OAI (Official Action Indicated), you must address the issues identified in the inspection report promptly. You can use platforms like Atlas Compliance to stay informed about potential risks and compliance trends.
Q4. How can AI tools help me manage FDA inspection outcomes?
A4. AI tools like Atlas Compliance use predictive analytics and machine learning models to identify potential compliance risks, helping you stay ahead of issues and maintain compliance with FDA regulations.