Companies in the pharmaceutical, biotech, and food industries must ensure their products remain consistently safe, effective, and compliant with public health standards. Maintaining this level of quality presents a significant challenge, as it requires rigorous oversight of every stage in the manufacturing process.
To meet these demands, manufacturers rely on Current Good Manufacturing Practices (cGMP). These regulatory guidelines, enforced by global authorities, provide a structured framework covering facility design, equipment maintenance, staff training, quality control, and documentation.
In this blog, we’ll explore what FDA cGMP involves, its essential components, and why strict compliance is vital to protecting both consumers and brand integrity.
TL;DR
- Overview of what cGMP is and why it’s critical to product quality and safety.
- cGMP compliance is legally mandated by the FDA and international regulators.
- Certification requires tight control over processes, personnel, and documentation.
- Non-compliance can lead to recalls, fines, or loss of manufacturing rights.
- Staying compliant protects consumers, brand integrity, and market access.
What is cGMP?
Current Good Manufacturing Practice (cGMP) refers to a set of regulations enforced by health authorities such as the U.S. Food and Drug Administration (FDA) and adopted internationally through bodies like the World Health Organization (WHO). These guidelines establish the minimum standards required for manufacturing processes to ensure products are consistently high in quality, safe for use, and free from contamination.
The emphasis on “current” highlights the need for manufacturers to continuously update their practices in line with the latest scientific advancements, technological improvements, and industry innovations.
In the U.S., cGMP regulations for human drugs are outlined in 21 CFR Parts 210 and 211 and updated through the Federal Register. Globally, authorities rely on similar frameworks like the WHO GMP guidelines and the EU’s EudraLex Volume 4 to guide inspections and ensure compliance.
Key Components of cGMP:
- Raw Material Sourcing: Only approved, high-quality materials are used to initiate the manufacturing process.
- Equipment Maintenance: Manufacturing equipment must be regularly calibrated and maintained to ensure precision and reliability.
- Personnel Training: All staff must be properly trained to maintain consistency and comply with standard procedures.
- Quality Control: Continuous testing and monitoring throughout production help identify and address issues early.
- Standard Operating Procedures (SOPs): Clear, detailed documentation ensures uniformity, traceability, and regulatory compliance.
The Core Objective: To embed quality at every stage of production, ensuring product integrity, consistency, and safety from start to finish.
Why is CGMP Certification Important in Pharmaceuticals?
Achieving cGMP (Current Good Manufacturing Practice) certification is crucial for pharmaceutical manufacturers, not only as a regulatory requirement but also as a foundation for product quality and global market credibility. Here’s why it holds such importance:
1. Assurance of Quality and Safety
The main purpose of FDA GMP is to safeguard the health and safety of consumers. cGMP standards ensure that medicines are consistently produced and controlled to meet quality standards, guaranteeing safety and effectiveness for patients.
2. Regulatory Compliance
Non-compliance with GMP regulations can lead to serious consequences, such as FDA warnings, fines, or even product recalls. In severe cases, companies may face legal action. Staying compliant with FDA GMP standards is essential to maintaining your company’s reputation and market access. In many countries, including the United States, European Union, Australia, and Japan, cGMP compliance is required for market authorization. Without it, pharmaceutical products cannot be legally sold in these regions.
3. Avoiding Inspection Failures
FDA inspections are a crucial part of the regulatory process. A failure to meet cGMP requirements can result in a Form 483 (a list of deficiencies) or a warning letter from the FDA. Both can lead to serious consequences, including loss of license to manufacture products. Preparing for these inspections and maintaining cGMP compliance is essential for smooth operations.
4. Maintaining Consumer Trust & Brand Reputation
Consumers trust the safety and efficacy of products they use. Adhering to cGMP not only ensures the quality of your products but also builds consumer confidence. A company with a strong cGMP track record is perceived as a reliable and trustworthy brand.
5. Global Market Access
Certification opens the door to international trade, particularly in highly regulated markets. Manufacturers who meet cGMP requirements are seen as credible and reliable partners worldwide.
For example, testing 100 tablets out of a batch of 2 million, the majority of the product is released to market without being individually tested. This makes it crucial that quality is built into every stage of the manufacturing process through strong cGMP controls. Rather than relying solely on end-product testing, cGMP ensures that quality, safety, and consistency are achieved through tightly controlled design, validated procedures, and vigilant oversight from the outset.
How to Get cGMP Certified: A Step-by-Step Guide
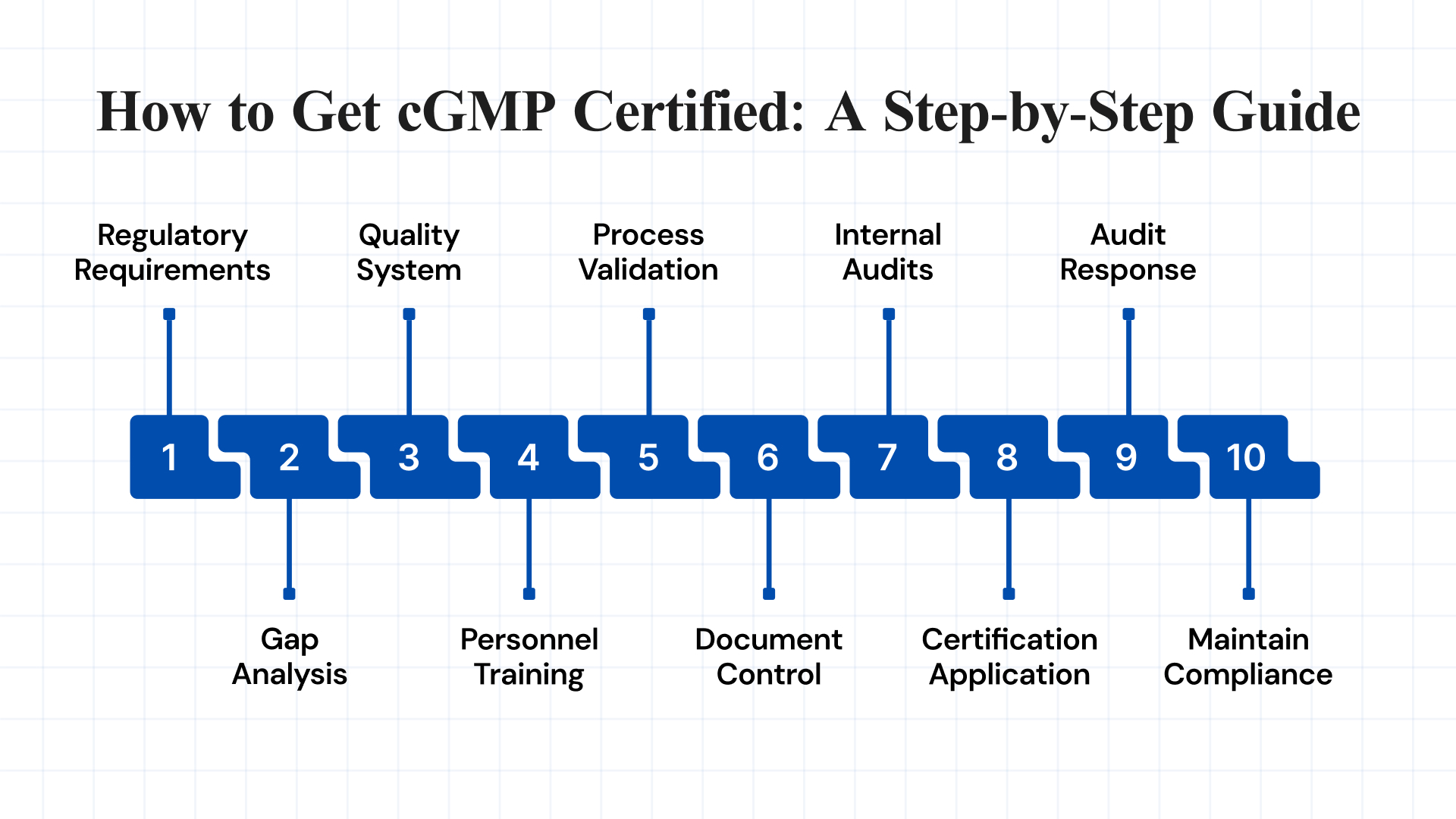
Achieving cGMP certification is a structured process that demonstrates your commitment to quality, safety, and regulatory compliance. Below is a step-by-step guide to help manufacturers prepare for and successfully obtain cGMP certification:
1. Understand Regulatory Requirements
Before pursuing cGMP certification, it’s essential to identify the specific regulations that apply to your product type and target market. Different regions have their own cGMP standards, including:
- 21 CFR Part 210 & 211: U.S. FDA regulations for drug manufacturing
- EU GMP Guidelines: Compliance requirements for the European Union
- WHO GMP: Globally accepted standards set by the World Health Organization
- ICH Q7, Q8, Q9, Q10: International guidelines for API manufacturing and pharmaceutical quality systems.
2. Perform a Comprehensive Gap Analysis
Assess current practices across departments to identify gaps in compliance with cGMP standards. Areas to review include:
- Facility layout and design
- Documentation and SOPs
- Staff training and records
- Equipment and process validation
- Quality assurance systems
- Data traceability and record-keeping
Document all gaps and resolve them using a CAPA (Corrective and Preventive Actions) system.
3. Build a Strong Quality Management System (QMS)
A compliant QMS is the foundation of cGMP. It should include:
- Well-defined SOPs for all operations
- A formal Quality Manual outlining quality policies
- Good documentation practices (following ALCOA principles)
- Systems for handling deviations, CAPAs, and change control
- Risk management for all quality-related processes
4. Train All Personnel on GMP Standards
Human error is a leading cause of GMP violations. Regular and role-specific training is critical. Core topics include:
- cGMP principles and updates
- Department-specific SOPs
- Hygiene, gowning, and contamination prevention
- Documentation, deviation handling, and CAPA protocols
Training should be documented and repeated periodically or when regulations change.
5. Validate All Processes and Equipment
Validation ensures consistent quality. Conduct the following qualifications, where applicable:
- Design Qualification (DQ): Ensures the design meets the intended use
- Installation Qualification (IQ): Confirms correct setup
- Operational Qualification (OQ): Verifies functional performance
- Performance Qualification (PQ): Confirms consistent operation under real conditions
This includes cleaning methods, sterilization, analytical testing, and computerized systems.
6. Implement Document Control and Record-Keeping Systems
Documentation is central to cGMP compliance. Maintain accurate, secure, and organized records for:
- Manufacturing and batch data
- Equipment usage and maintenance
- Training records
- Calibration logs and audit findings
Ensure all documentation follows ALCOA principles—Attributable, Legible, Contemporaneous, Original, and Accurate.
7. Conduct Internal Audits (Self-Inspections)
Internal audits simulate regulatory inspections and help uncover hidden gaps. Include:
- Facility and equipment checks
- SOP and documentation reviews
- Staff interviews and training evaluations
- Quality checks and CAPA effectiveness
Audits should be cross-functional and performed by trained staff from other departments.
8. Submit Certification Application
Once you’re confident in your facility’s compliance, apply to the relevant certification body, such as:
- FDA: For U.S. market access
- EU GMP: For the European Union
- ISO/WHO: For international recognition
- Local health authorities – As per your country’s requirements
The audit will assess facility operations, staff knowledge, and all documentation.
9. Respond to Audit Findings Promptly
Post-inspection, regulators may issue observations (e.g., FDA 483s, WHO or EU non-compliance reports). You must:
- Analyze root causes
- Submit a CAPA plan within the timeline
- Implement actions and track progress
- Maintain follow-up documentation for re-inspection if required
10. Maintain Certification and Continuous Compliance
Certification is not a one-time achievement. Ongoing efforts are required to remain compliant:
- Conduct regular self-inspections
- Update SOPs and training materials
- Monitor regulatory updates
- Encourage a company-wide culture of quality and compliance
Being audit-ready at all times is the key to sustained success.
What Are Some Practical Examples of cGMP?
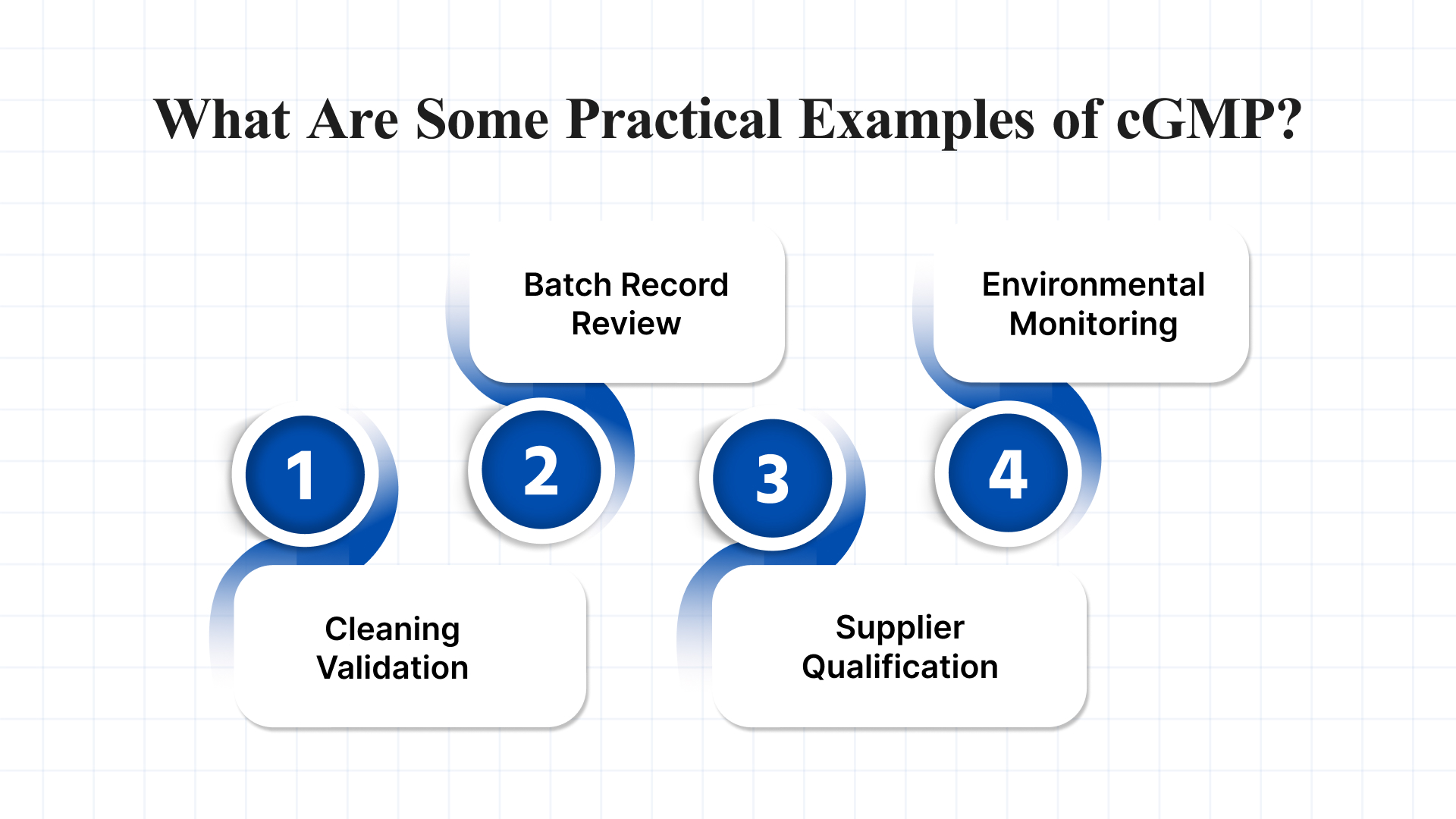
Here are a few key examples of how cGMP principles are applied in real-world manufacturing settings:
1. Cleaning Validation
What it means: Cleaning validation ensures that the cleaning procedures used for equipment and manufacturing environments effectively remove residues of products, cleaning agents, and microbial contaminants.
Real-world example: In a pharmaceutical company that manufactures both antibiotics and pain relievers on shared equipment, cleaning validation is critical to prevent cross-contamination. After producing an antibiotic batch, the company runs a validated cleaning cycle and then swabs the equipment for chemical and microbiological residues. Lab tests confirm that all traces are below the allowable limits before the next product (e.g., paracetamol) is manufactured. This prevents unintended exposure and protects patient safety.
2. Batch Record Review
What it means: Every manufactured batch must have a corresponding batch record that documents each step of the process, from raw materials to final packaging. Reviewing these records ensures compliance and helps identify any deviations or errors.
Real-world example: Before releasing a batch of insulin pens to the market, a Quality Assurance (QA) team at a biotech facility reviews the batch record. They verify that all ingredients were used within their expiry dates, that the fill volume was consistent, and that temperature logs during cold storage were within acceptable ranges. A discrepancy in a packaging label is caught during review, allowing for correction before distribution, avoiding a potential product recall.
3. Supplier Qualification
What it means: Manufacturers must assess and approve all suppliers of raw materials, components, and services to ensure that incoming goods meet required quality standards.
Real-world example: A dietary supplement company sources herbal extracts from multiple global vendors. Before approving a new supplier in India, the quality team audits their facility, checks historical test results, and confirms the consistency of active ingredient levels in sample shipments. Only after passing this evaluation is the supplier added to the approved vendor list. This process prevents substandard or adulterated ingredients from entering production.
4. Environmental Monitoring
What it means: Regular testing of air, surfaces, and water in cleanroom areas ensures that the manufacturing environment meets strict cleanliness standards to prevent contamination, especially in sterile product manufacturing.
Real-world example: In a sterile injectable manufacturing facility, operators must work inside cleanrooms where even a few airborne particles can jeopardize product sterility. The facility uses particle counters and microbial air samplers daily. When a spike in airborne microorganisms is detected near a filling line, production is paused, the area is disinfected, and root cause analysis is performed. This proactive monitoring prevents compromised products from reaching patients.
These real-world examples highlight how cGMP principles are not just guidelines. They are critical operational practices that help ensure product safety, consistency, and compliance with regulatory standards.
Common Challenges in cGMP Compliance and How to Overcome Them
Achieving and maintaining cGMP compliance comes with several recurring challenges that can impact product quality and regulatory standing. Here are common issues and effective ways to overcome them.
| Challenge | Description | How to Overcome |
| Data Integrity | Ensuring accuracy, reliability, and security of both electronic and paper records. | Conduct regular audits; train staff on ALCOA principles and data handling best practices. |
| Change Control | Managing updates to equipment, processes, or systems while ensuring traceability. | Implement a structured change control system; involve cross-functional teams for approval and review. |
| Regulatory Updates | Keeping up with changing local and global cGMP requirements. | Subscribe to regulatory alerts, attend industry conferences, and engage in professional forums/networks. |
To effectively address these challenges, companies need more than manual oversight; they require advanced tools that streamline compliance and improve operational control.
How Atlas Compliance Helps You Stay cGMP-Compliant?
Staying compliant with evolving FDA GMP regulations requires precision, awareness, and continuous improvement. Atlas Compliance simplifies this challenge by equipping pharmaceutical manufacturers and other regulated industries with advanced, AI-powered tools that keep operations aligned with cGMP standards.
Unique Features Include:
- Site Profiles Across GLP, GCP, GDP, and GMP: Atlas Compliance creates comprehensive profiles that span all key compliance domains, giving your team a clear view of performance and risk across your entire organization.
- Structured Compliance Intelligence from Unstructured Data: By organizing and analyzing unstructured compliance data, Atlas facilitates a shift from reactive responses to proactive decision-making, thereby reducing risk exposure and enhancing operational productivity.
Core Capabilities:
- FDA Inspection Intelligence: Access a robust database of FDA inspection reports, including 483s, warning letters, and CFR citations. This intelligence helps identify common compliance pitfalls and align your practices with FDA expectations.
- AI-Powered Document Search: Advanced NLP-driven tools allow your team to instantly locate relevant information in regulatory documents, SOPs, and audit reports, saving time and reducing the risk of oversight.
- Predictive Compliance Analytics: Machine learning analyzes historical inspection data to identify patterns and forecast potential risks. These insights support preventative planning and continuous quality improvement.
- Supplier Risk Management: Ensure that your suppliers meet regulatory standards through integrated risk scoring, monitoring, and audit readiness tools, protecting your supply chain from non-compliance threats.
- Regulatory Surveillance: Stay ahead of regulatory updates with real-time monitoring of FDA guidelines, inspection trends, and industry developments, keeping your quality systems responsive and up to date.
Conclusion
Achieving and maintaining cGMP certification is not just a regulatory obligation, but it’s a commitment to product quality, consumer safety, and business sustainability. With strict global standards, complex inspection procedures, and constantly evolving regulations, pharmaceutical and life sciences companies need more than manual processes to stay ahead.
The solution lies in combining a strong internal quality system with intelligent, digital tools that simplify compliance management. Platforms like Atlas Complianceoffer an integrated approach, bringing together FDA inspection data, predictive analytics, AI-powered document search, and supplier oversight to help you stay inspection-ready at all times.
Book a free demo with us today and discover how we can improve your cGMP foundation and equip your team with the right tools.
FAQs
1. What is the difference between FDA GMP and cGMP?
The primary distinction lies in the term “current.” While “GMP” refers to general manufacturing practices, “cGMP” emphasizes the “current” standards that incorporate the latest technological advancements and regulatory requirements. This ensures that manufacturing processes are continuously updated to maintain product quality and safety.
2. How does the FDA determine if a company is complying with cGMP regulations?
The FDA conducts inspections of pharmaceutical manufacturing facilities worldwide, including those that manufacture active ingredients and finished products. These inspections follow a standard approach and are conducted by highly trained FDA staff. Additionally, the FDA relies on reports from the public and the industry of potentially defective drug products to identify sites for inspection or investigation.
3. What are the consequences of non-compliance with cGMP?
Failure to comply with cGMP regulations can result in serious consequences, including the distribution of adulterated drugs. Such drugs may not meet safety, identity, strength, quality, or purity standards. The FDA may take regulatory actions such as issuing warning letters, imposing fines, or even seizing products to protect public health.
4. Are there exemptions from cGMP requirements?
Generally, all drug manufacturers must adhere to cGMP regulations. However, certain exemptions may apply based on specific circumstances, such as the scale of production or the nature of the product. It’s essential for manufacturers to consult with regulatory experts or the FDA to understand any applicable exemptions.
5. What role does data integrity play in cGMP compliance?
Data integrity is a critical component of cGMP compliance. The FDA expects all data to be reliable and accurate, as lapses in data integrity can lead to significant regulatory actions. Manufacturers should implement meaningful and effective strategies to manage data integrity risks, ensuring that all data is complete, consistent, and accurate throughout its lifecycle.