When a defect or nonconformity arises in a product, it can disrupt supply chains, impact customer trust, and lead to significant operational delays. One of the key challenges businesses face is not just identifying these issues, but ensuring they are resolved at the root level and prevented from recurring.
This is where a Corrective Action Request (CAR) becomes essential. A CAR is a formal request issued to a supplier or manufacturer, requiring them to investigate the cause of a specific nonconformity or defect. It serves as a structured method to drive accountability, identify the root cause, and implement corrective measures.
By following the CAR process, organizations can improve product quality, improve supplier performance, and reduce the risk of repeated issues, ultimately supporting a more reliable and efficient operation.
TL;DR
- What a CAR is and how it works.
- The key benefits CARs offer to organizations include improved quality, compliance, and operational efficiency.
- A step-by-step guide for implementing an effective CAR process.
- Common challenges in implementing CAR and how to overcome them.
- Best practices for effective Corrective Action Request implementation to ensure lasting impact.
What is a Corrective Action Request?
A Corrective Action Request is a key component of an effective Quality Management System (QMS), widely used in industries such as manufacturing, defense, and regulated sectors. It is a formal process initiated to address nonconformities, deviations, or failures to meet established quality standards or regulatory requirements.
- Ensures product and process conformance to quality and regulatory standards.
- Helps improve functionality and overall performance.
- Reduces the risk of future non-compliance.
- Enables organizations to identify the root causes of quality issues.
- Facilitates the implementation of effective corrective actions to prevent recurrence.
CARs in Quality and Defense Standards
CAR and ISO 9001: Corrective Action Requests play a key role in ISO 9001 compliance. The standard emphasizes continuous improvement, and CARs help organizations meet this by formally addressing nonconformities and preventing recurrence. CARs are central to a strong Quality Management System (QMS), making them indispensable for ISO-certified businesses.
DCMA and Defense Contracts: In defense contracting, the Defense Contract Management Agency (DCMA) uses CARs to ensure suppliers meet contractual quality requirements. These requests help maintain accountability and traceability when resolving nonconformities in mission-critical or safety-sensitive defense systems.
Corrective Action Request (CAR) Levels
The U.S. Department of Defense (DoD) classifies Corrective Action Requests (CARs) into four levels, based on the severity of the nonconformity and the degree of management involvement required to resolve the issue. Here’s a breakdown of each level:
- Level I: Issued for nonconformities that can be immediately resolved on-site, with no need for a formal corrective action response. Level I CARs should still be documented and communicated to the appropriate supplier management responsible for addressing the issue.
- Level II: Used when nonconformities, particularly those involving Critical Safety Item (CSI) characteristics or Safety of Flight (SOF) concerns, cannot be resolved on the spot. These CARs are issued to the appropriate level of supplier management responsible for initiating corrective actions.
- Level III: Directed to the supplier’s top management for serious or repeated nonconformities, especially single-point failure SOF issues identified within a one-year period. These CARs may be accompanied by contractual consequences such as withholding progress payments, disallowing costs, or revoking business system approvals. Unlike lower levels, a Level III CAR can be issued independently of prior Level I or II notices and must be coordinated with the Contracting Officer.
- Level IV: Reserved for the most critical cases, either when a Level III CAR has failed to bring resolution, or when the nonconformity is so severe that immediate contractual remedies are necessary. These remedies may include suspending product acceptance or payments, in line with FAR/DFARS regulations. Level IV CARs are escalated to the supplier’s executive leadership.
Key Components of the Corrective Action Request Process
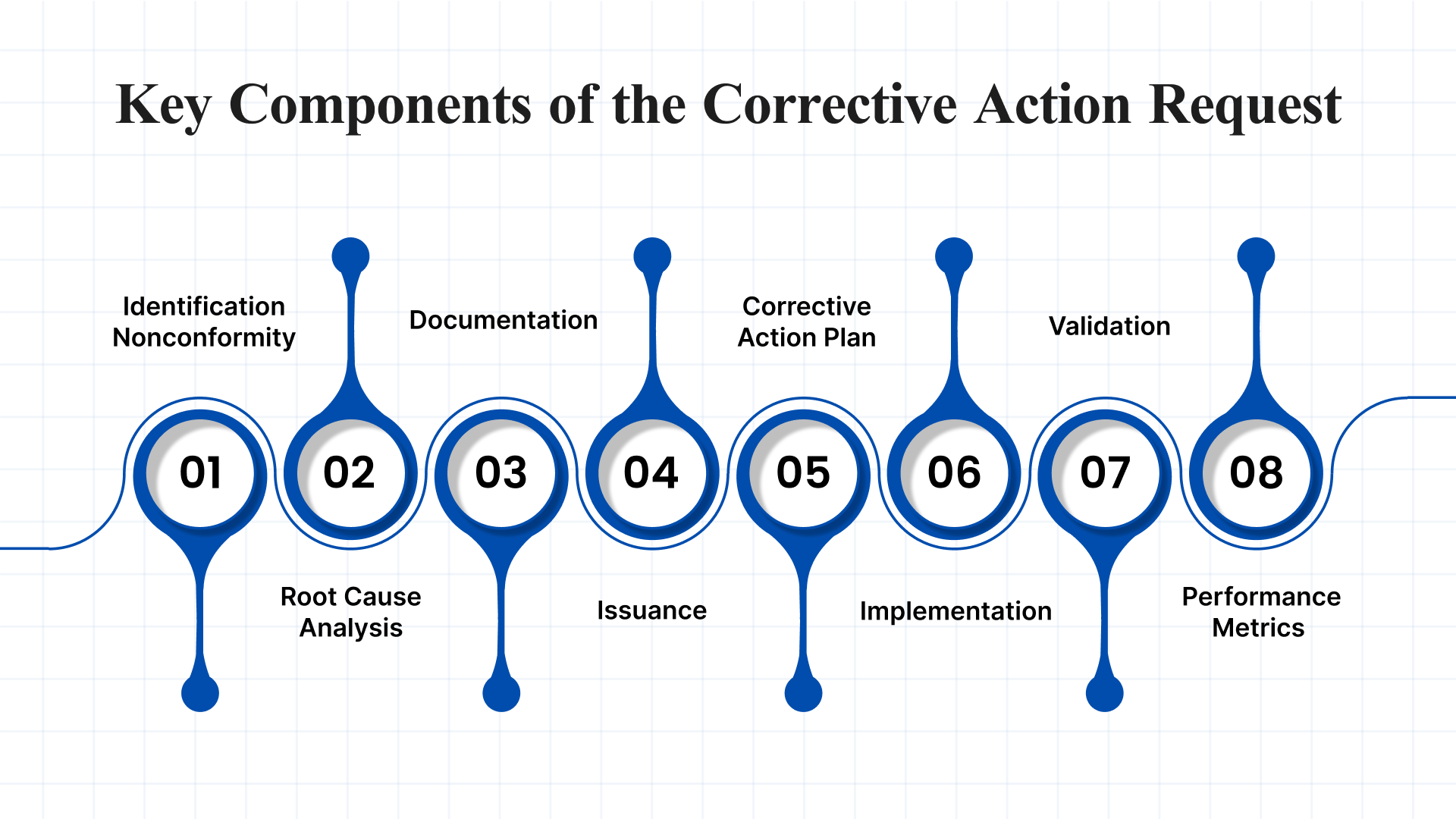
A Corrective Action Request (CAR) is a structured approach used by organizations to identify, resolve, and prevent quality or compliance issues.
- Identification of Nonconformity: The process begins with detecting a nonconformity or compliance issue, typically uncovered during quality inspections, audits, or routine evaluations.
- Root Cause Analysis: A critical step in the CAR process involves a structured investigation to determine the underlying reason for the nonconformity. This helps prevent recurrence and strengthens future quality assurance efforts.
- Documentation: The issue is formally recorded using a CAR form or template, which includes details such as the nature of the nonconformity, its potential impact, and findings from the root cause analysis.
- Issuance: Once the issue has been identified and documented, the CAR is formally issued to the appropriate team or department responsible for addressing the problem.
- Corrective Action Plan: The assigned team must develop a comprehensive corrective action plan, detailing how the issue will be resolved, addressing the root cause, and providing clear timelines for completion.
- Implementation: The corrective actions are then carried out, which may involve modifying processes, reworking defective products, or introducing new controls to prevent recurrence.
- Validation: After implementation, the actions taken are validated to ensure the issue has been fully resolved and the process or product meets required quality standards.
- Follow-Up and Performance Metrics: Ongoing monitoring is conducted to ensure the corrective measures remain effective over time. Key performance indicators and metrics are tracked to evaluate the success and sustainability of the CAR process.
Atlas Compliance simplifies the management of corrective and preventive action (CAPA) plans by offering advanced tools for streamlined documentation and tracking. Utilizing its extensive database of past FDA inspections, the platform allows organizations to benchmark their plans against industry standards and expected outcomes, ensuring each action plan thoroughly addresses root causes and prevents future issues.
Steps in Developing a Corrective Action Request
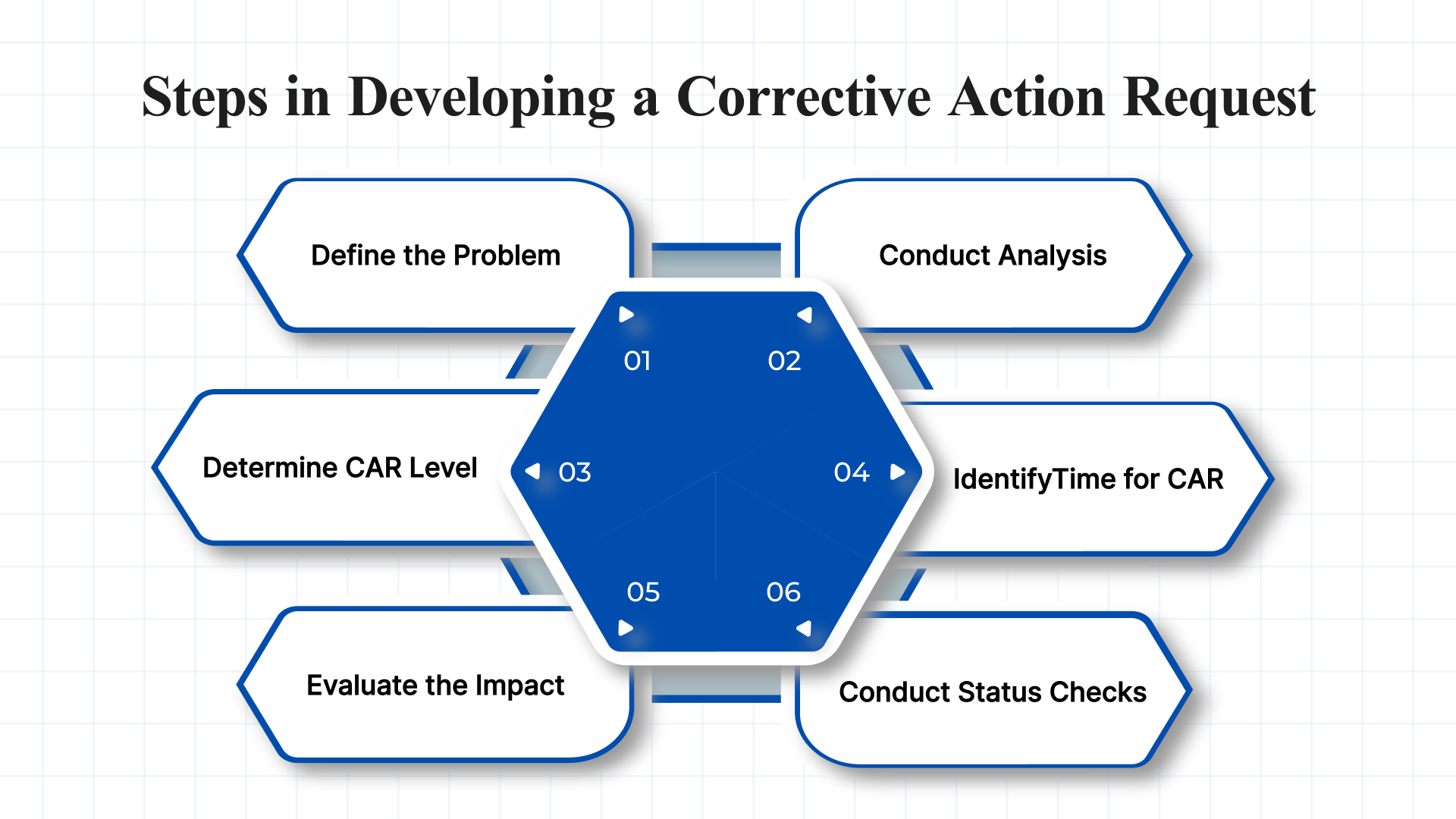
Developing an effective Corrective Action Request involves a structured, step-by-step approach to ensure nonconformities are thoroughly addressed and prevented from recurring. Below are the seven essential steps to implement a robust CAR process:
1. Clearly Define the Problem and the Required Corrective Actions
Begin by identifying and describing the nonconformity or issue in detail. Be specific about what went wrong, when it occurred, and where it was found. This helps establish a clear understanding of the problem and guides the corrective actions needed to resolve it. Ensure the issue is documented with supporting evidence and specify the quality standards or requirements that were not met.
2. Conduct a Root Cause Analysis
Once the issue is defined, perform a thorough root cause analysis to uncover the underlying cause(s) of the problem, not just the symptoms. Techniques like the “5 Whys,” Fishbone Diagram (Ishikawa), or Failure Mode and Effects Analysis (FMEA) can be used to trace the source of the issue systematically. Accurate root cause identification is critical to implementing an effective and lasting solution.
3. Determine the Appropriate CAR Level
Not all issues require the same level of response. Assess the severity, frequency, and impact by evaluating factors such as safety implications, operational disruption, and compliance risks to select the correct CAR level aligned with management involvement. For example, minor issues may only require Level I action, while recurring or critical issues might necessitate Level III or IV attention.
4. Identify the Right Time to Issue a CAR
Timing is crucial in addressing nonconformities. Issue the CAR as soon as the problem is clearly understood and sufficiently documented. Delaying the process can allow the issue to worsen or recur. Issuing the CAR promptly allows corrective actions to be implemented before the problem has a broader impact on quality, safety, compliance, or customer satisfaction.
5. Evaluate the Impact on Cost, Schedule, and Performance
Understand how the nonconformity and its resolution will affect the overall project or operational metrics. Assess potential delays, rework costs, or impact on product quality and delivery. This information helps prioritize the CAR and allocate the appropriate level of resources and urgency needed for resolution.
6. Conduct Regular CAR Status Checks
Once the corrective action plan is in place and being executed, perform regular status checks to monitor progress. Ensure milestones are being met, corrective measures are effective, and responsible parties are fulfilling their roles. Periodic reviews involve scheduled meetings to assess progress against milestones, address any obstacles encountered, and reassign resources as needed to maintain accountability throughout the CAR lifecycle.
Corrective Action Request Examples
Corrective Action Requests (CARs) are commonly used across a wide range of industries, including manufacturing, healthcare, and finance, to resolve nonconformities and improve processes.
- Healthcare Sector: In healthcare, CARs are implemented to address critical patient safety concerns such as medical errors, adverse events, or near-miss incidents. By identifying the root cause and developing a targeted corrective action plan, healthcare providers can enhance the quality of care, improve safety protocols, and prevent similar incidents from occurring again.
- Financial Sector: In the financial industry, CARs help manage compliance issues such as regulatory breaches or internal control weaknesses. Initiating a CAR allows institutions to thoroughly investigate the root cause, implement corrective actions, and reinforce compliance measures, ultimately minimizing the risk of future violations.
Overcoming CAR Implementation Challenges
Implementing an effective Corrective Action Request (CAR) process is critical for ensuring quality, compliance, and continuous improvement. However, many organizations face practical and systemic challenges that can undermine the process if not addressed proactively.
| Challenge | Problem | Solution |
|---|---|---|
| Weak Root Cause Analysis | Focus on symptoms, not causes | Use tools like 5 Whys, Fishbone Diagram |
| Poor Documentation | Incomplete or unclear CAR records | Standardize forms, use digital tracking tools |
| Slow Response | Delays in resolving issues | Set deadlines, assign owners, automate alerts |
| Low Stakeholder Involvement | Limited team input | Involve cross-functional teams early |
| Lack of Training | Staff unclear on CAR process | Regular training on tools & procedures |
| Change Resistance | Pushback on process changes | Communicate value, build quality-first culture |
| No Follow-Up | Actions not tracked or verified | Schedule checks, use KPIs to monitor outcomes |
| Limited Resources | Time, staff, or budget constraints | Prioritize critical issues, automate where possible |
| Low Leadership Support | Management doesn’t prioritize CARs | Include CARs in KPIs and performance reviews |
| Tech Integration Issues | Legacy systems disrupt CAR flow | Use integrated QMS or AI tools like Atlas |
Best Practices for Effective Corrective Action Request Implementation
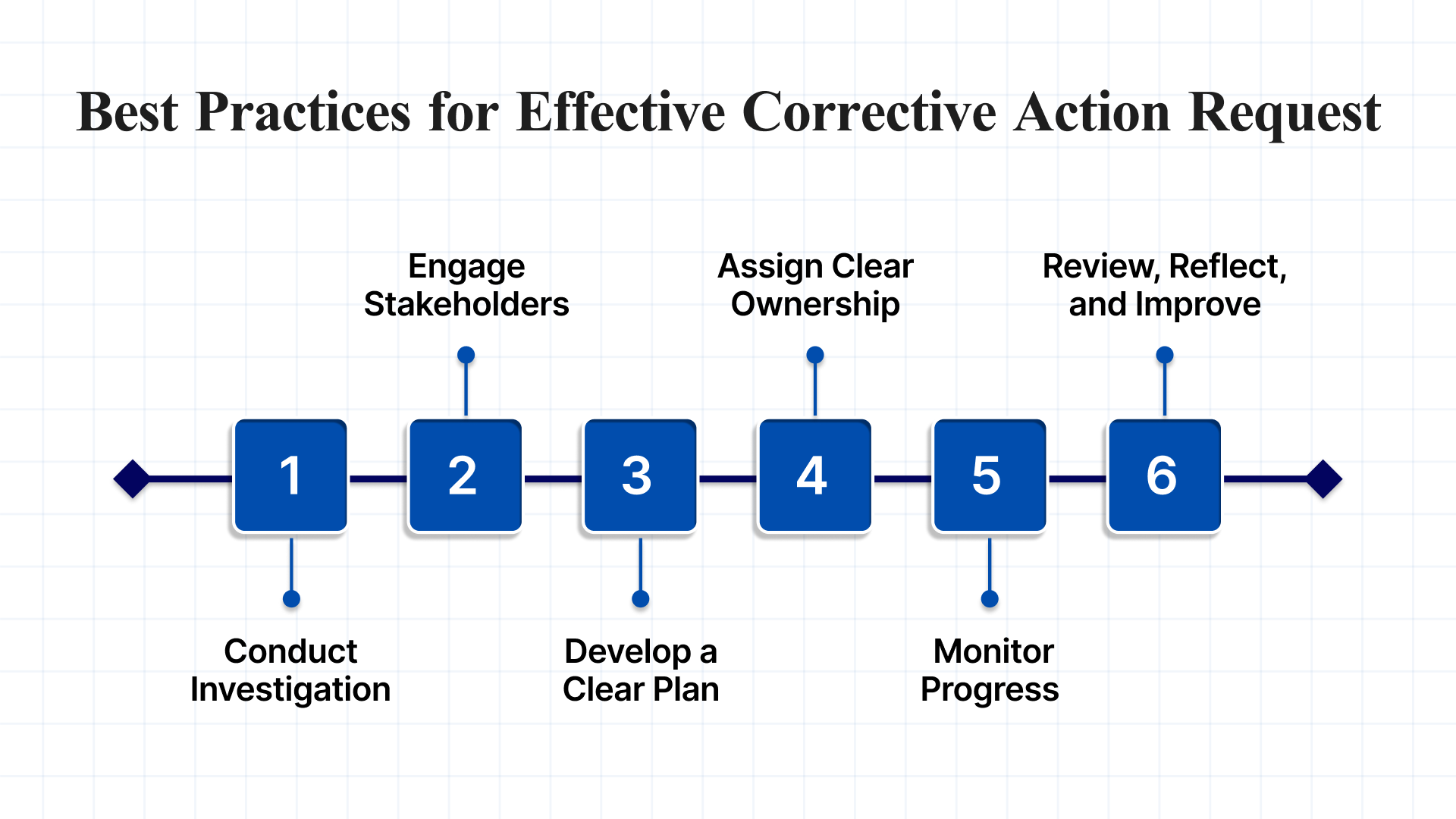
Implementing a Corrective Action Request effectively begins with preparation. Manufacturers should start by creating a comprehensive Supplier Corrective Action Request (SCAR) form that captures all necessary data to support a root cause analysis. In parallel, it’s essential to establish a well-defined CAR procedure. Below are key best practices to guide successful implementation:
1. Conduct a Thorough Investigation
To develop a meaningful CAR, begin with a detailed investigation. Gather relevant data, assess the full scope of the issue, and perform a root cause analysis. This ensures that the corrective action targets the source of the problem—not just the symptoms—resulting in a more permanent solution.
2. Engage Relevant Stakeholders
Include all impacted stakeholders in the CAR process to ensure a holistic view of the issue. By involving departments and individuals affected by the nonconformity, you gain valuable insights and foster collaboration. Stakeholder input also helps shape a more effective and widely accepted corrective action plan.
3. Develop a Clear, Actionable Plan
A successful CAR requires a detailed action plan. This plan should outline:
- The responsible party for implementation
- A defined timeline for each task
- Necessary resources
- Specific action steps to be taken
Clarity in the action plan ensures alignment, accountability, and smoother execution.
4. Assign Clear Ownership
Designate an owner for each CAR who is accountable for its execution. This person oversees progress, resolves obstacles, and ensures timely completion. Establish clear expectations and communication protocols to support consistent updates and effective problem-solving.
5. Monitor Progress and Conduct Follow-Ups
Track each stage of the CAR implementation against the action plan. Monitor progress regularly, identify delays or barriers, and perform follow-up checks to confirm that the issue has been effectively resolved and does not recur.
6. Review, Reflect, and Improve
Once the CAR is closed, conduct a review of the entire process. Evaluate what worked well and what could be improved. Document lessons learned and incorporate those insights into future quality processes to enhance organizational resilience and prevent repeat issues.
You can also check out this blog to know more about SCAR: Understanding Supplier Corrective Action Request (SCAR) Process
How Atlas Compliance Can Help in the Corrective Action Request (CAR) Process
Atlas Compliance is an AI-powered platform that improves every stage of the Corrective Action Request (CAR) process, offering pharmaceutical companies and regulated industries robust tools to manage nonconformances and maintain compliance effectively.
From identifying issues to tracking corrective actions and ensuring resolution, Atlas streamlines and reinforces the entire CAR workflow.
Here’s how Atlas-Compliance.ai supports the CAR process:
- Predictive Analytics for Risk Identification: Utilizes machine learning, Atlas analyzes historical inspection and quality data to identify emerging risks and supplier nonconformities early. This proactive approach reduces the likelihood of future CARs by addressing potential issues before they escalate.
- AI-Powered Document Search and Management: By integrating Natural Language Processing (NLP), Atlas enables teams to quickly locate relevant information within large regulatory documents, such as FDA inspection reports. This accelerates documentation processes and improves accuracy when generating and managing CARs.
- Centralized CAR Documentation and Tracking: Atlas offers a unified platform to log and monitor CARs from initiation through closure. It captures all related data, including corrective actions, supplier communications, and approval statuses, ensuring transparency, audit readiness, and streamlined follow-up.
- Real-Time Regulatory Surveillance: The platform actively monitors FDA updates and industry regulations to ensure your CAR process remains compliant with evolving standards and best practices.
- Supplier Risk Management: It involves analyzing supplier audit results, delivery performance, and compliance records using data analytics to identify and mitigate potential risks proactively.
- Streamlined CAPA Integration: The tool enhances CAPA (Corrective and Preventive Action) development by providing structured workflows for documenting, implementing, and verifying corrective actions. Predictive analytics also helps validate the long-term effectiveness of those actions.
Conclusion
Corrective Action Requests are an essential part of maintaining regulatory compliance and product quality in industries regulated by bodies such as the FDA. By following a systematic CAR process, identifying root causes, and implementing robust corrective actions, you can reduce the risk of recurring issues and maintain a high level of operational excellence.
Moreover, at Atlas Compliance, we offer AI-powered tools that simplify and streamline the CAR process. From predictive analytics to continuous regulatory surveillance, we provide organizations with comprehensive insights into FDA inspections, helping companies stay compliant and audit-ready.
Looking to streamline your CAR process? See how Atlas Compliance can keep you compliant and always prepared for inspections. Book a free demo today!
FAQs
Q1. What is an example of a Corrective Action Request (CAR)?
A1. An example of a CAR might occur when an audit identifies that a manufacturing process is failing to meet quality standards. In response, the corrective action could involve retraining staff on standard operating procedures or updating and correcting the relevant process documentation.
Q2. What is a Level 3 Corrective Action Request?
A2. A Level III CAR is directed to a supplier’s top management to address serious contractual nonconformities. It is typically issued when there is a failure to resolve a Level II CAR or when the same issue recurs, indicating ineffective root cause analysis and insufficient corrective action at previous levels.
Q3. What are the four steps to complete a Corrective Action Request?
A3. 1. Issue Identification: Capture and record the issue in the corrective action system.
2. Root Cause Analysis: Investigate the problem thoroughly to identify its root cause.
3. Solution Implementation: Develop and apply corrective actions to resolve the issue.
4. Effectiveness Review: Evaluate and verify that the corrective actions have resolved the issue and prevented recurrence.
Q4. What is a major Corrective Action Request?
A4. A major CAR addresses significant issues that impact product or process quality, functionality, or regulatory compliance. These requests are critical for identifying the root causes of nonconformities and implementing strong corrective measures to ensure ongoing compliance and quality assurance.
